Interleukin-8 blockade prevents activated endothelial cell mediated proliferation and chemoresistance of acute myeloid leukemia
- PMID: 31299413
- PMCID: PMC6857733
- DOI: 10.1016/j.leukres.2019.106180
Interleukin-8 blockade prevents activated endothelial cell mediated proliferation and chemoresistance of acute myeloid leukemia
Abstract
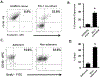
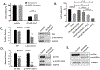
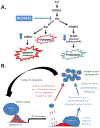
One of the greatest challenges in treating acute myeloid leukemia (AML) is chemotherapy refractory disease. Previously, we demonstrated a novel mechanism whereby AML-induced endothelial cell (EC) activation leads to subsequent leukemia cell adherence, quiescence and chemoresistance, identifying activated ECs as potential mediators of relapse. We now show mechanistically that EC activation induces the secretion of interleukin-8 (IL-8) leading to significant expansion of non-adherent AML cells and resistance to cytarabine (Ara-C). Through crystallography and computational modeling, we identified a pocket within IL-8 responsible for receptor binding, screened for small molecules that fit within this pocket, and blocked IL-8 induced proliferation and chemo-protection of AML cells with a hit compound. Results from this study show a new therapeutic strategy for targeting the sanctuary of an activated leukemia microenvironment.
Keywords: Acute myeloid leukemia; Chemoresistance; Endothelial cell; Interleukin-8; Microenvironment; Vascular niche.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures


References
-
- Tallman MS, Gilliland DG, Rowe JM, Drug therapy for acute myeloid leukemia, Blood 106(4) (2005) 1154–63. - PubMed
-
- Doan PL, Chute JP, The vascular niche: home for normal and malignant hematopoietic stem cells, Leukemia 26(1) (2012) 54–62. - PubMed
-
- Kiel MJ, Radice GL, Morrison SJ, Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance, Cell Stem Cell 1(2) (2007) 204–17. - PubMed
-
- Cogle CR, Goldman DC, Madlambayan GJ, Leon RP, Al Masri A, Clark HA, Asbaghi SA, Tyner JW, Dunlap J, Fan G, Kovacsovics T, Liu Q, Meacham A, Hamlin KL, Hromas RA, Scott EW, Fleming WH, Functional integration of acute myeloid leukemia into the vascular niche, Leukemia 28(10) (2014) 1978–87. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

