The oncolytic efficacy and safety of avian reovirus and its dynamic distribution in infected mice
- PMID: 31299861
- PMCID: PMC6879773
- DOI: 10.1177/1535370219861928
The oncolytic efficacy and safety of avian reovirus and its dynamic distribution in infected mice
Abstract
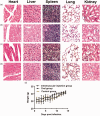
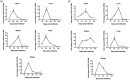
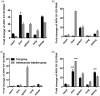
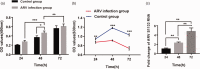
Primary liver cancer is a major public health challenge that ranks as the third most common cause of cancer worldwide despite therapeutic improvement. Reovirus has been emerging as a potential anti-cancer agent and is undergoing multiple clinical trials, and it is reported that reovirus can preferentially cause the cell death of a variety of cancers in a manner of apoptosis. As few studies have reported the efficacy of oncolytic activity and safety profile of avian reovirus, in our study, LDH assay, MTT assay, DAPI staining, and flow cytometry assay were performed to demonstrate the oncolytic effects of avian reovirus against the HepG2 cells, and quantitative real-time PCR (qRT-PCR) and animal experiments were conducted to investigate the dynamic distribution of avian reovirus in infected mice and then illustrate the safety and tissue tropism of avian reovirus. LDH assay, DAPI staining, and flow cytometry assay confirmed the efficacy of the oncotherapeutic effects of avian reovirus, and MTT assay has indicated that avian reovirus suppressed the proliferation of HepG2 cells and decreased their viability significantly. qRT-PCR revealed the dynamic distribution of avian reovirus in infected mice that avian reovirus might replicate better and have more powerful oncolytic activity in liver, kidney, and spleen tissues. Furthermore, histopathological examination clearly supported that avian reovirus appeared non-pathogenic to the normal host, so our study may provide the new insights and rationale for the new strategy of removing liver cancer.
Impact statement: We demonstrated the efficacy of oncolytic activity of avian reovirus (ARV) by LDH assay, MTT assay, DAPI staining, and flow cytometry assay, and also investigated the dynamic distribution of ARV in infected mice and then illustrated the safety and tissue tropism of ARV by quantitative real-time PCR (qRT-PCR) and animal experiments. Collectively, our study may provide the new insights and rationale for the new strategy of removing liver cancer.
Keywords: Hepatocellular carcinoma; avian reovirus; dynamic distribution; oncolytic virus; real-time PCR; safety.
Figures

References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics. CA Cancer J Clin 2015; 65:87–108 - PubMed
-
- Vucenik I, Zhang ZS, Shamsuddin AM. IP6 in treatment of liver cancer II. Intra-tumoral injection of IP6 regresses pre-existing human liver cancer xenotransplanted in nude mice. Anticancer Res 1998; 18:4091–6 - PubMed
-
- Kowdley KV, Hassanein T, Kaur S, Farrell FJ, Van Thiel DH, Keeffe EB, Sorrell MF, Bacon BR, Weber FJ, Tavill AS. Primary liver cancer and survival in patients undergoing liver transplantation for hemochromatosis. Liver Transpl Surg 1995; 1:237–41 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

