Pulsed-laser irradiation of multifunctional gold nanoshells to overcome trastuzumab resistance in HER2-overexpressing breast cancer
- PMID: 31299997
- PMCID: PMC6626398
- DOI: 10.1186/s13046-019-1305-x
Pulsed-laser irradiation of multifunctional gold nanoshells to overcome trastuzumab resistance in HER2-overexpressing breast cancer
Abstract
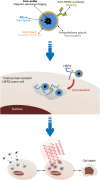
Background: HER2-overexpressing metastatic breast cancers are challenging practice in oncology when they become resistant to anti-HER2 therapies such as trastuzumab. In these clinical situations, HER2-overexpression persists in metastatic localizations, and can thus be used for active targeting using innovative therapeutic approaches. Functionalized gold nanoparticles with anti-HER2 antibody can be stimulated by near-infrared light to induce hyperthermia.
Methods: Here, hybrid anti-HER2 gold nanoshells were engineered for photothermal therapy to overcome trastuzumab resistance in HER2-overexpressing breast cancer xenografts.
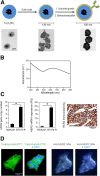
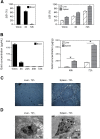
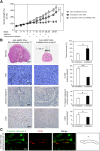
Results: When gold nanoshells were administered in HER2-tumor xenografts, no toxicity was observed. A detailed pharmacokinetic study showed a time-dependent accumulation of gold nanoshells within the tumors, significantly greater with functionalized gold nanoshells at 72 h. This enabled us to optimize the treatment protocol and irradiate the mice when the anti-HER2 gold nanoshells had accumulated most in the tumors. After weekly injections of anti-HER2 gold nanoshells, and repeated irradiations with a femtosecond-pulsed laser over four weeks, tumor growth was significantly inhibited. Detailed tissue microscopic analyses showed that the tumor growth inhibition was due to an anti-angiogenic effect, coherent with a preferential distribution of the nanoshells in tumor microvessels. We also showed a direct tumor cell effect with apoptosis and inhibition of proliferation, coherent with an immune-mediated targeting of tumor cells by anti-HER2 nanoshells.
Conclusion: This preclinical study thus supports the use of anti-HER2 gold nanoshells and photothermal therapy to overcome trastuzumab resistance in HER2-overexpressing breast cancer.
Keywords: Femtosecond laser; Functionalized gold nanoparticles; HER2-overexpressing breast cancer; Photothermal therapy; Resistance reversion; Trastuzumab resistance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Immunoconjugated gold nanoshell-mediated photothermal ablation of trastuzumab-resistant breast cancer cells.Breast Cancer Res Treat. 2011 Jan;125(1):27-34. doi: 10.1007/s10549-010-0811-5. Epub 2010 Mar 10. Breast Cancer Res Treat. 2011. PMID: 20217215
-
Photothermal therapeutic application of gold nanorods-porphyrin-trastuzumab complexes in HER2-positive breast cancer.Sci Rep. 2017 Feb 3;7:42069. doi: 10.1038/srep42069. Sci Rep. 2017. PMID: 28155894 Free PMC article.
-
Inhibiting HER3 Hyperphosphorylation in HER2-Overexpressing Breast Cancer through Multimodal Therapy with Branched Gold Nanoshells.Small. 2023 Dec;19(50):e2303934. doi: 10.1002/smll.202303934. Epub 2023 Aug 26. Small. 2023. PMID: 37632323
-
Novel targeted therapies to overcome trastuzumab resistance in HER2-overexpressing metastatic breast cancer.Curr Drug Targets. 2013 Jul;14(8):889-98. doi: 10.2174/13894501113149990161. Curr Drug Targets. 2013. PMID: 23531110 Review.
-
Recent Insights into the Development of Preclinical Trastuzumab- Resistant HER2+ Breast Cancer Models.Curr Med Chem. 2018;25(17):1976-1998. doi: 10.2174/0929867323666161216144659. Curr Med Chem. 2018. PMID: 27993109 Review.
Cited by
-
Research progress in tumor angiogenesis and drug resistance in breast cancer.Cancer Biol Med. 2024 Jun 25;21(7):571-85. doi: 10.20892/j.issn.2095-3941.2023.0515. Cancer Biol Med. 2024. PMID: 38940663 Free PMC article. Review.
-
Designing the Surface Chemistry of Inorganic Nanocrystals for Cancer Imaging and Therapy.Cancers (Basel). 2022 May 16;14(10):2456. doi: 10.3390/cancers14102456. Cancers (Basel). 2022. PMID: 35626059 Free PMC article. Review.
-
Targeting Cancer Stem Cells to Overcome Chemoresistance.Int J Mol Sci. 2018 Dec 13;19(12):4036. doi: 10.3390/ijms19124036. Int J Mol Sci. 2018. PMID: 30551640 Free PMC article. Review.
-
How to Make Anticancer Drugs Cross the Blood-Brain Barrier to Treat Brain Metastases.Int J Mol Sci. 2019 Dec 18;21(1):22. doi: 10.3390/ijms21010022. Int J Mol Sci. 2019. PMID: 31861465 Free PMC article. Review.
-
Brain Metastasis Treatment: The Place of Tyrosine Kinase Inhibitors and How to Facilitate Their Diffusion across the Blood-Brain Barrier.Pharmaceutics. 2021 Sep 10;13(9):1446. doi: 10.3390/pharmaceutics13091446. Pharmaceutics. 2021. PMID: 34575525 Free PMC article. Review.
References
-
- Grabar KC, Allison KJ, Baker BE, Bright RM, Brown KR, Freeman RG, et al. J Langmuir. 1996;12:2353. doi: 10.1021/la950561h. - DOI
-
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–2648. doi: 10.1200/JCO.1999.17.9.2639. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

