Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma - results of a prospective biomarker study
- PMID: 31300034
- PMCID: PMC6625062
- DOI: 10.1186/s40425-019-0659-0
Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma - results of a prospective biomarker study
Abstract
Background: Metastasized or unresectable melanoma has been the first malignant tumor to be successfully treated with checkpoint inhibitors. Nevertheless, about 40-50% of the patients do not respond to these treatments and severe side effects are observed in up to 60%. Therefore, there is a high need to identify reliable biomarkers predicting response. Tumor Mutation Burden (TMB) is a debated predictor for response to checkpoint inhibitors and early measurement of ctDNA can help to detect treatment failure to immunotherapy in selected melanoma patients. However, it has not yet been clarified how TMB and ctDNA can be used to estimate response to combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma.
Patients and methods: In this prospective biomarker study, we included 35 melanoma patients with ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1) therapy. In all patients, a tumor panel of 710 tumor-associated genes was applied (tumor vs. reference tissue comparison), followed by repetitive liquid biopsies. Cell-free DNA was extracted and at least one driver mutation was monitored. Treatment response was evaluated after about three months of therapy.
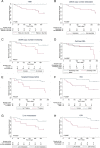
Results: TMB was significantly higher in responders than in nonresponders and TMB > 23.1 Mut/Mb (TMB-high) was associated with a survival benefit compared to TMB ≤ 23.1 Mut/Mb (TMB-low or TMB-intermediate). Furthermore, a > 50% decrease of cell-free DNA concentration or undetectable circulating tumor DNA (ctDNA), measured by tumor-specific variant copies/ml of plasma at first follow-up three weeks after treatment initiation were significantly associated with response to combined immunotherapy and improved overall survival, respectively. It is noticeable that no patient with TMB ≤ 23.1 Mut/Mb and detectable or increasing ctDNA at first follow-up responded to immunotherapy.
Conclusion: High TMB, > 50% decrease of cell-free DNA concentration, and undetectable ctDNA at first follow-up seem to be associated with response and overall survival under combined immunotherapy. The evaluation of ctDNA and cell-free DNA three weeks after treatment initiation may be suitable for early assessment of efficacy of immunotherapy.
Conflict of interest statement
AF served as consultant to Roche, Novartis, MSD, Pierre-Fabre; received travel support from Roche, Novartis, BMS, Pierre-Fabre, received speaker fees from Cegat, Roche, Novartis, BMS, MSD. TA received travel support from Novartis. TE served as consultant to Roche, Novartis, MSD, BMS, Pierre-Fabre; received speaker fees from Roche, Novartis, BMS, MSD. CG reports grants and personal fees from Novartis, BMS, Roche, personal fees from MSD. Personal fees from Amgen, Philogen, LEO, Incyte, outside the submitted work. DD received travel support from BMS. No competing interests were declared by the other authors.
Figures



References
-
- Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–1492. doi: 10.1016/S1470-2045(18)30700-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
