Acid-Base Basics
- PMID: 31300088
- PMCID: PMC7836985
- DOI: 10.1016/j.semnephrol.2019.04.002
Acid-Base Basics
Erratum in
-
Erratum to "Acid-Base Basics" (Semin Nephrol. 2019;39:316-27).Semin Nephrol. 2019 Sep;39(5):515. doi: 10.1016/j.semnephrol.2019.07.001. Semin Nephrol. 2019. PMID: 31514915 No abstract available.
Abstract
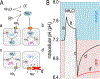
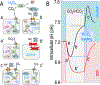
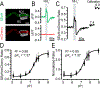
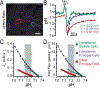
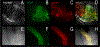
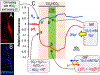
Although students initially learn of ionic buffering in basic chemistry, buffering and acid-base transport in biology often is relegated to specialized classes, discussions, or situations. That said, for physiology, nephrology, pulmonology, and anesthesiology, these basic principles often are critically important for mechanistic understanding, medical treatments, and assessing therapy effectiveness. This short introductory perspective focuses on basic chemistry and transport of buffers and acid-base equivalents, provides an outline of basic science acid-base concepts, tools used to monitor intracellular pH, model cellular responses to pH buffer changes, and the more recent development and use of genetically encoded pH-indicators. Examples of newer genetically encoded pH-indicators (pHerry and pHire) are provided, and their use for in vitro, ex vivo, and in vivo experiments are described. The continued use and development of these basic tools provide increasing opportunities for both basic and potentially clinical investigations.
Keywords: CO(2)/HCO(3)(-) buffering; GEpHI; Intracellular pH; ammonium pulse; genetically encoded pH indicator; pH buffering.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement: M.F.R. is employed by the Mayo Clinic Foundation and has received federal funding to support this area of research.
Figures
References
-
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–8. - PubMed
-
- Busa WB, Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984;246:R409–38. - PubMed
-
- Trivedi B, Danforth WH. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem. 1966;241:4110–2. - PubMed
-
- Pouyssegur J, Franchi A, L’Allemain G, Paris S. Cytoplasmic pH, a key determinant of growth factor-induced DNA synthesis in quiescent fibroblasts. FEBS Lett. 1985;190:115–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

