RDH10 function is necessary for spontaneous fetal mouth movement that facilitates palate shelf elevation
- PMID: 31300413
- PMCID: PMC6679383
- DOI: 10.1242/dmm.039073
RDH10 function is necessary for spontaneous fetal mouth movement that facilitates palate shelf elevation
Abstract
Cleft palate is a common birth defect, occurring in approximately 1 in 1000 live births worldwide. Known etiological mechanisms of cleft palate include defects within developing palate shelf tissues, defects in mandibular growth and defects in spontaneous fetal mouth movement. Until now, experimental studies directly documenting fetal mouth immobility as an underlying cause of cleft palate have been limited to models lacking neurotransmission. This study extends the range of anomalies directly demonstrated to have fetal mouth movement defects correlated with cleft palate. Here, we show that mouse embryos deficient in retinoic acid (RA) have mispatterned pharyngeal nerves and skeletal elements that block spontaneous fetal mouth movement in utero Using X-ray microtomography, in utero ultrasound video, ex vivo culture and tissue staining, we demonstrate that proper retinoid signaling and pharyngeal patterning are crucial for the fetal mouth movement needed for palate formation. Embryos with deficient retinoid signaling were generated by stage-specific inactivation of retinol dehydrogenase 10 (Rdh10), a gene crucial for the production of RA during embryogenesis. The finding that cleft palate in retinoid deficiency results from a lack of fetal mouth movement might help elucidate cleft palate etiology and improve early diagnosis in human disorders involving defects of pharyngeal development.
Keywords: Cleft palate; RDH10; Retinoic acid; Spontaneous mouth movement; Vitamin A.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

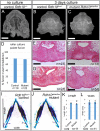

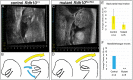


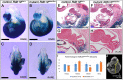

Similar articles
-
Mesenchymal changes associated with retinoic acid induced cleft palate in CD-1 mice.J Craniofac Genet Dev Biol. 1998 Apr-Jun;18(2):88-99. J Craniofac Genet Dev Biol. 1998. PMID: 9672841
-
Modulating Wnt Signaling Rescues Palate Morphogenesis in Pax9 Mutant Mice.J Dent Res. 2017 Oct;96(11):1273-1281. doi: 10.1177/0022034517719865. Epub 2017 Jul 10. J Dent Res. 2017. PMID: 28692808 Free PMC article.
-
Experimental induction of palate shelf elevation in glutamate decarboxylase 67-deficient mice with cleft palate due to vertically oriented palatal shelf.Birth Defects Res A Clin Mol Teratol. 2007 Oct;79(10):688-95. doi: 10.1002/bdra.20400. Birth Defects Res A Clin Mol Teratol. 2007. PMID: 17849453
-
Roles of retinoic acid signaling in normal and abnormal development of the palate and tongue.Congenit Anom (Kyoto). 2014 May;54(2):69-76. doi: 10.1111/cga.12049. Congenit Anom (Kyoto). 2014. PMID: 24666225 Review.
-
The etiopathogenesis of cleft lip and cleft palate: usefulness and caveats of mouse models.Curr Top Dev Biol. 2008;84:37-138. doi: 10.1016/S0070-2153(08)00602-9. Curr Top Dev Biol. 2008. PMID: 19186243 Review.
Cited by
-
Celebrating FocalPlane and microscopy in Disease Models & Mechanisms.Dis Model Mech. 2021 Jul 1;14(7):dmm049183. doi: 10.1242/dmm.049183. Epub 2021 Jul 19. Dis Model Mech. 2021. PMID: 34279567 Free PMC article. No abstract available.
-
Divergent growth of the transient brain compartments in fetuses with nonsyndromic isolated clefts involving the primary and secondary palate.Cereb Cortex. 2024 Jan 31;34(2):bhae024. doi: 10.1093/cercor/bhae024. Cereb Cortex. 2024. PMID: 38365268 Free PMC article.
-
Clinical Approach to Cleft Lip and Palate with or Without Surgical Correction in Ten Brachycephalic Puppies.Vet Sci. 2025 May 14;12(5):474. doi: 10.3390/vetsci12050474. Vet Sci. 2025. PMID: 40431567 Free PMC article.
-
Three-dimensional reconstruction of systematic histological sections: application to observations on palatal shelf elevation.Int J Oral Sci. 2021 May 26;13(1):17. doi: 10.1038/s41368-021-00122-8. Int J Oral Sci. 2021. PMID: 34039957 Free PMC article.
-
A Systematic Review of Oropharyngeal Dysphagia Models in Rodents.Int J Environ Res Public Health. 2021 May 7;18(9):4987. doi: 10.3390/ijerph18094987. Int J Environ Res Public Health. 2021. PMID: 34067192 Free PMC article.
References
-
- Asada H., Kawamura Y., Maruyama K., Kume H., Ding R.-G., Kanbara N., Kuzume H., Sanbo M., Yagi T. and Obata K. (1997). Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc. Natl. Acad. Sci. USA 94, 6496-6499. 10.1073/pnas.94.12.6496 - DOI - PMC - PubMed
-
- Barrow J. R. and Capecchi M. R. (1999). Compensatory defects associated with mutations in Hoxa1 restore normal palatogenesis to Hoxa2 mutants. Development 126, 5011-5026. - PubMed
-
- Bel-Vialar S., Itasaki N. and Krumlauf R. (2002). Initiating Hox gene expression: in the early chick neural tube differential sensitivity to FGF and RA signaling subdivides the HoxB genes in two distinct groups. Development 129, 5103-5115. - PubMed
-
- Burdi A. R. and Faist K. (1967). Morphogenesis of the palate in normal human embryos with special emphasis on the mechanisms involved. Am. J. Anat. 120, 149-159. 10.1002/aja.1001200112 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

