Tetrodotoxin-Sensitive Sodium Channels Mediate Action Potential Firing and Excitability in Menthol-Sensitive Vglut3-Lineage Sensory Neurons
- PMID: 31300524
- PMCID: PMC6733551
- DOI: 10.1523/JNEUROSCI.2817-18.2019
Tetrodotoxin-Sensitive Sodium Channels Mediate Action Potential Firing and Excitability in Menthol-Sensitive Vglut3-Lineage Sensory Neurons
Abstract
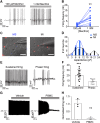
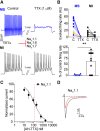
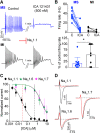
Small-diameter vesicular glutamate transporter 3-lineage (Vglut3lineage) dorsal root ganglion (DRG) neurons play an important role in mechanosensation and thermal hypersensitivity; however, little is known about their intrinsic electrical properties. We therefore set out to investigate mechanisms of excitability within this population. Calcium microfluorimetry analysis of male and female mouse DRG neurons demonstrated that the cooling compound menthol selectively activates a subset of Vglut3lineage neurons. Whole-cell recordings showed that small-diameter Vglut3lineage DRG neurons fire menthol-evoked action potentials and exhibited robust, transient receptor potential melastatin 8 (TRPM8)-dependent discharges at room temperature. This heightened excitability was confirmed by current-clamp and action potential phase-plot analyses, which showed menthol-sensitive Vglut3lineage neurons to have more depolarized membrane potentials, lower firing thresholds, and higher evoked firing frequencies compared with menthol-insensitive Vglut3lineage neurons. A biophysical analysis revealed voltage-gated sodium channel (NaV) currents in menthol-sensitive Vglut3lineage neurons were resistant to entry into slow inactivation compared with menthol-insensitive neurons. Multiplex in situ hybridization showed similar distributions of tetrodotoxin (TTX)-sensitive NaV transcripts between TRPM8-positive and -negative Vglut3lineage neurons; however, NaV1.8 transcripts, which encode TTX-resistant channels, were more prevalent in TRPM8-negative neurons. Conversely, pharmacological analyses identified distinct functional contributions of NaV subunits, with NaV1.1 driving firing in menthol-sensitive neurons, whereas other small-diameter Vglut3lineage neurons rely primarily on TTX-resistant NaV channels. Additionally, when NaV1.1 channels were blocked, the remaining NaV current readily entered into slow inactivation in menthol-sensitive Vglut3lineage neurons. Thus, these data demonstrate that TTX-sensitive NaVs drive action potential firing in menthol-sensitive sensory neurons and contribute to their heightened excitability.SIGNIFICANCE STATEMENT Somatosensory neurons encode various sensory modalities including thermoreception, mechanoreception, nociception, and itch. This report identifies a previously unknown requirement for tetrodotoxin-sensitive sodium channels in action potential firing in a discrete subpopulation of small-diameter sensory neurons that are activated by the cooling agent menthol. Together, our results provide a mechanistic understanding of factors that control intrinsic excitability in functionally distinct subsets of peripheral neurons. Furthermore, as menthol has been used for centuries as an analgesic and anti-pruritic, these findings support the viability of NaV1.1 as a therapeutic target for sensory disorders.
Keywords: action potential; dorsal root ganglion; excitability; sensory neuron; sodium channel.
Copyright © 2019 the authors.
Figures







Similar articles
-
Sodium channel diversity in the vestibular ganglion: NaV1.5, NaV1.8, and tetrodotoxin-sensitive currents.J Neurophysiol. 2016 May 1;115(5):2536-55. doi: 10.1152/jn.00902.2015. Epub 2016 Mar 2. J Neurophysiol. 2016. PMID: 26936982 Free PMC article.
-
Tetrodotoxin-sensitive voltage-gated sodium channels regulate bladder afferent responses to distension.Pain. 2018 Dec;159(12):2573-2584. doi: 10.1097/j.pain.0000000000001368. Pain. 2018. PMID: 30157135
-
Tetrodotoxin-resistant sodium channels in sensory neurons generate slow resurgent currents that are enhanced by inflammatory mediators.J Neurosci. 2014 May 21;34(21):7190-7. doi: 10.1523/JNEUROSCI.5011-13.2014. J Neurosci. 2014. PMID: 24849353 Free PMC article.
-
Targeting voltage gated sodium channels NaV1.7, Na V1.8, and Na V1.9 for treatment of pathological cough.Lung. 2014 Feb;192(1):15-20. doi: 10.1007/s00408-013-9533-x. Epub 2013 Nov 24. Lung. 2014. PMID: 24272479 Free PMC article. Review.
-
Tetrodotoxin, a Potential Drug for Neuropathic and Cancer Pain Relief?Toxins (Basel). 2021 Jul 12;13(7):483. doi: 10.3390/toxins13070483. Toxins (Basel). 2021. PMID: 34357955 Free PMC article. Review.
Cited by
-
CaMKII enhances voltage-gated sodium channel Nav1.6 activity and neuronal excitability.J Biol Chem. 2020 Aug 14;295(33):11845-11865. doi: 10.1074/jbc.RA120.014062. Epub 2020 Jul 1. J Biol Chem. 2020. PMID: 32611770 Free PMC article.
-
Chronic morphine regulates TRPM8 channels via MOR-PKCβ signaling.Mol Brain. 2020 Apr 14;13(1):61. doi: 10.1186/s13041-020-00599-0. Mol Brain. 2020. PMID: 32290846 Free PMC article.
-
Differential encoding of mammalian proprioception by voltage-gated sodium channels.Sci Adv. 2025 Jan 10;11(2):eads6660. doi: 10.1126/sciadv.ads6660. Epub 2025 Jan 8. Sci Adv. 2025. PMID: 39772670 Free PMC article.
-
Intrinsic adaptive plasticity in mouse and human sensory neurons.J Gen Physiol. 2025 Jan 6;157(1):e202313488. doi: 10.1085/jgp.202313488. Epub 2024 Dec 17. J Gen Physiol. 2025. PMID: 39688836 Free PMC article.
-
The Electrically Silent Kv5.1 Subunit Forms Heteromeric Kv2 Channels in Cortical Neurons and Confers Distinct Functional Properties.J Neurosci. 2025 Mar 26;45(13):e2293232025. doi: 10.1523/JNEUROSCI.2293-23.2025. J Neurosci. 2025. PMID: 39933932
References
-
- Alexandrou AJ, Brown AR, Chapman ML, Estacion M, Turner J, Mis MA, Wilbrey A, Payne EC, Gutteridge A, Cox PJ, Doyle R, Printzenhoff D, Lin Z, Marron BE, West C, Swain NA, Storer RI, Stupple PA, Castle NA, Hounshell JA, et al. (2016) Subtype-selective small molecule inhibitors reveal a fundamental role for Nav1.7 in nociceptor electrogenesis, axonal conduction and presynaptic release. PLoS One 11:e0152405. 10.1371/journal.pone.0152405 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
