Cardiomyocyte orientation modulated by the Numb family proteins-N-cadherin axis is essential for ventricular wall morphogenesis
- PMID: 31300538
- PMCID: PMC6681736
- DOI: 10.1073/pnas.1904684116
Cardiomyocyte orientation modulated by the Numb family proteins-N-cadherin axis is essential for ventricular wall morphogenesis
Abstract
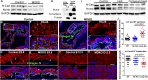
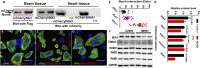
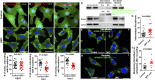
The roles of cellular orientation during trabecular and ventricular wall morphogenesis are unknown, and so are the underlying mechanisms that regulate cellular orientation. Myocardial-specific Numb and Numblike double-knockout (MDKO) hearts display a variety of defects, including in cellular orientation, patterns of mitotic spindle orientation, trabeculation, and ventricular compaction. Furthermore, Numb- and Numblike-null cardiomyocytes exhibit cellular behaviors distinct from those of control cells during trabecular morphogenesis based on single-cell lineage tracing. We investigated how Numb regulates cellular orientation and behaviors and determined that N-cadherin levels and membrane localization are reduced in MDKO hearts. To determine how Numb regulates N-cadherin membrane localization, we generated an mCherry:Numb knockin line and found that Numb localized to diverse endocytic organelles but mainly to the recycling endosome. Consistent with this localization, cardiomyocytes in MDKO did not display defects in N-cadherin internalization but rather in postendocytic recycling to the plasma membrane. Furthermore, N-cadherin overexpression via a mosaic model partially rescued the defects in cellular orientation and trabeculation of MDKO hearts. Our study unravels a phenomenon that cardiomyocytes display spatiotemporal cellular orientation during ventricular wall morphogenesis, and its disruption leads to abnormal trabecular and ventricular wall morphogenesis. Furthermore, we established a mechanism by which Numb modulates cellular orientation and consequently trabecular and ventricular wall morphogenesis by regulating N-cadherin recycling to the plasma membrane.
Keywords: Numb family proteins; cellular orientation; endocytosis; single-cell lineage tracing; trabecular morphogenesis.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Olson E. N., A decade of discoveries in cardiac biology. Nat. Med. 10, 467–474 (2004). - PubMed
-
- Manasek F. J., Embryonic development of the heart. I. A light and electron microscopic study of myocardial development in the early chick embryo. J. Morphol. 125, 329–365 (1968). - PubMed
-
- Van Mierop L. H., Embryology of the univentricular heart. Herz 4, 78–85 (1979). - PubMed
-
- Sedmera D., Pexieder T., Vuillemin M., Thompson R. P., Anderson R. H., Developmental patterning of the myocardium. Anat. Rec. 258, 319–337 (2000). - PubMed
-
- Icardo J. M., Fernandez-Terán A., Morphologic study of ventricular trabeculation in the embryonic chick heart. Acta Anat. (Basel) 130, 264–274 (1987). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

