Hereditary tyrosinemia type I-associated mutations in fumarylacetoacetate hydrolase reduce the enzyme stability and increase its aggregation rate
- PMID: 31300554
- PMCID: PMC6721957
- DOI: 10.1074/jbc.RA119.009367
Hereditary tyrosinemia type I-associated mutations in fumarylacetoacetate hydrolase reduce the enzyme stability and increase its aggregation rate
Abstract
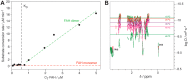
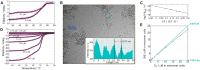
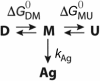
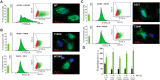
More than 100 mutations in the gene encoding fumarylacetoacetate hydrolase (FAH) cause hereditary tyrosinemia type I (HT1), a metabolic disorder characterized by elevated blood levels of tyrosine. Some of these mutations are known to decrease FAH catalytic activity, but the mechanisms of FAH mutation-induced pathogenicity remain poorly understood. Here, using diffusion ordered NMR spectroscopy, cryo-EM, and CD analyses, along with site-directed mutagenesis, enzymatic assays, and molecular dynamics simulations, we investigated the putative role of thermodynamic and kinetic stability in WT FAH and a representative set of 19 missense mutations identified in individuals with HT1. We found that at physiological temperatures and concentrations, WT FAH is in equilibrium between a catalytically active dimer and a monomeric species, with the latter being inactive and prone to oligomerization and aggregation. We also found that the majority of the deleterious mutations reduce the kinetic stability of the enzyme and always accelerate the FAH aggregation pathway. Depending mainly on the position of the amino acid in the structure, pathogenic mutations either reduced the dimer population or decreased the energy barrier that separates the monomer from the aggregate. The mechanistic insights reported here pave the way for the development of pharmacological chaperones that target FAH to tackle the severe disease HT1.
Keywords: biophysics; enzyme mutation; fumaryl acetoacetate hydrolase; nuclear magnetic resonance (NMR); protein aggregation; protein stability; rare disease; tyrosine; tyrosinemia type I.
© 2019 Macias et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Cao Y. Y., Zhang Y. L., Du J., Qu Y. J., Zhong X. M., Bai J. L., and Song F. (2012) Compound mutations (R237X and L375P) in the fumarylacetoacetate hydrolase gene causing tyrosinemia type I in a Chinese patient. Chin. Med. J. (Engl.) 125, 2132–2136 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

