Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes
- PMID: 31300673
- PMCID: PMC6626021
- DOI: 10.1038/s41467-019-11139-3
Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes
Abstract
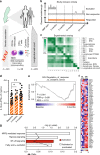
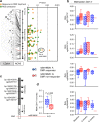
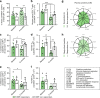
Dimethyl fumarate (DMF) is a first-line-treatment for relapsing-remitting multiple sclerosis (RRMS). The redox master regulator Nrf2, essential for redox balance, is a target of DMF, but its precise therapeutic mechanisms of action remain elusive. Here we show impact of DMF on circulating monocytes and T cells in a prospective longitudinal RRMS patient cohort. DMF increases the level of oxidized isoprostanes in peripheral blood. Other observed changes, including methylome and transcriptome profiles, occur in monocytes prior to T cells. Importantly, monocyte counts and monocytic ROS increase following DMF and distinguish patients with beneficial treatment-response from non-responders. A single nucleotide polymorphism in the ROS-generating NOX3 gene is associated with beneficial DMF treatment-response. Our data implicate monocyte-derived oxidative processes in autoimmune diseases and their treatment, and identify NOX3 genetic variant, monocyte counts and redox state as parameters potentially useful to inform clinical decisions on DMF therapy of RRMS.
Conflict of interest statement
F.P. has received research grants from Biogen, Novartis, and Genzyme, and fees for serving as Chair of DMC in clinical trials with Parexel. T.O. has received unrestricted MS research grants, and/or lecture advisory board honoraria from Biogen, Novartis, Sanofi and Roche. The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

