In vivo two-photon microscopy of the human eye
- PMID: 31300680
- PMCID: PMC6626016
- DOI: 10.1038/s41598-019-46568-z
In vivo two-photon microscopy of the human eye
Abstract
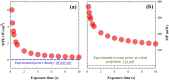
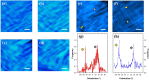
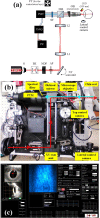
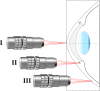
Two-photon (2P) microscopy is a powerful tool for imaging and exploring label-free biological tissues at high resolution. Although this type of microscopy has been demonstrated in ex vivo ocular tissues of both humans and animal models, imaging the human eye in vivo has always been challenging. This work presents a novel compact 2P microscope for non-contact imaging of the anterior part of the living human eye. The performance of the instrument was tested and the maximum permissible exposure to protect ocular tissues established. To the best of our knowledge, 2P images of the in vivo human cornea, the sclera and the trabecular meshwork are shown for the very first time. Acquired images are of enough quality to visualize collagen arrangement and morphological features of clinical interest. Future implementations of this technique may constitute a potential tool for early diagnosis of ocular diseases at submicron scale.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

