Differential effects of slow rewarming after cerebral hypothermia on white matter recovery after global cerebral ischemia in near-term fetal sheep
- PMID: 31300687
- PMCID: PMC6626025
- DOI: 10.1038/s41598-019-46505-0
Differential effects of slow rewarming after cerebral hypothermia on white matter recovery after global cerebral ischemia in near-term fetal sheep
Abstract
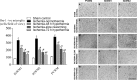
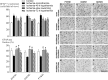
It is widely believed that rewarming slowly after therapeutic hypothermia for hypoxic-ischemic (HI) encephalopathy can improve outcomes, but its impact on white matter injury after HI is unclear. Fetal sheep (0.85 gestation) received 30 min ischemia-normothermia (n = 8), or hypothermia from 3-48 h with rapid spontaneous rewarming over 1 h (ischemia-48 h hypothermia, n = 8), or 48 h with slow rewarming over 24 h (ischemia-slow rewarming, n = 7) or 72 h with rapid rewarming (ischemia-72 h hypothermia, n = 8). Ischemia was associated with loss of total and mature oligodendrocytes and reduced area fraction of myelin basic protein (MBP) and 2',3'-cyclic nucleotide 3'-phosphodiesterase (CNPase; immature/mature oligodendrocytes) and increased microglia and astrocytes. Total numbers of oligodendrocytes were increased by all hypothermia protocols but only ischemia-72 h hypothermia attenuated loss of mature oligodendrocytes. All hypothermia protocols similarly increased the area fraction of MBP, whereas there was only an intermediate effect on the area fraction of CNPase. Microglia were suppressed by all hypothermia protocols, with the greatest reduction after ischemia-72 h hypothermia, and an intermediate effect after ischemia-slow rewarming. By contrast, induction of astrocytes was significantly reduced only after ischemia-slow rewarming. In conclusion, slow rewarming after hypothermia did not improve oligodendrocyte survival or myelination or suppression of microgliosis compared to fast rewarming, but modestly reduced astrocytosis.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

