Pre-existing Antineuraminidase Antibodies Are Associated With Shortened Duration of Influenza A(H1N1)pdm Virus Shedding and Illness in Naturally Infected Adults
- PMID: 31300819
- PMCID: PMC7245146
- DOI: 10.1093/cid/ciz639
Pre-existing Antineuraminidase Antibodies Are Associated With Shortened Duration of Influenza A(H1N1)pdm Virus Shedding and Illness in Naturally Infected Adults
Abstract
Background: Influenza causes a substantial burden worldwide, and current seasonal influenza vaccine has suboptimal effectiveness. To develop better, more broadly protective vaccines, a more thorough understanding is needed of how antibodies that target the influenza virus surface antigens, hemagglutinin (HA) (including head and stalk regions) and neuraminidase (NA), impact influenza illness and virus transmission.
Methods: We used a case-ascertained, community-based study of household influenza virus transmission set in Managua, Nicaragua. Using data from 170 reverse transcriptase-polymerase chain reaction (RT-PCR)-confirmed influenza virus A(H1N1)pdm infections and 45 household members with serologically confirmed infection, we examined the association of pre-existing NA, hemagglutination inhibiting, and HA stalk antibody levels and influenza viral shedding and disease duration using accelerated failure time models.
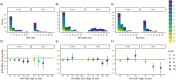
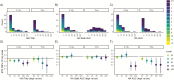
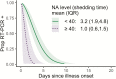
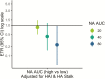
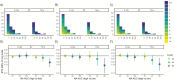
Results: Among RT-PCR-confirmed infections in adults, pre-existing anti-NA antibody levels ≥40 were associated with a 69% (95% confidence interval [CI], 34-85%) shortened shedding duration (mean, 1.0 vs 3.2 days). Neuraminidase antibody levels ≥80 were associated with further shortened shedding and significantly shortened symptom duration (influenza-like illness, 82%; 95% CI, 39-95%). Among RT-PCR-confirmed infections in children, hemagglutination inhibition titers ≥1:20 were associated with a 32% (95% CI, 13-47%) shortened shedding duration (mean, 3.9 vs 6.0 days).
Conclusions: Our results suggest that anti-NA antibodies play a large role in reducing influenza illness duration in adults and may impact transmission, most clearly among adults. Neuraminidase should be considered as an additional target in next-generation influenza virus vaccine development.We found that antibodies against neuraminidase were associated with significantly shortened viral shedding, and among adults they were also associated with shortened symptom duration. These results support neuraminidase as a potential target of next-generation influenza virus vaccines.
Keywords: influenza; hemagglutination inhibition (HAI); household; neuraminidase; shedding.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- World Health Organization. Influenza (seasonal). Available at: http://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal). Accessed 15 October 2018.
-
- Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2004–2018 | seasonal influenza (Flu) | CDC 2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed 15 October 2018.
-
- Paules CI, Marston HD, Eisinger RW, Baltimore D, Fauci AS. The pathway to a universal influenza vaccine. Immunity 2017; 47:599–603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

