Patterns of Drug and Alcohol Use and Injection Equipment Sharing Among People With Recent Injecting Drug Use or Receiving Opioid Agonist Treatment During and Following Hepatitis C Virus Treatment With Direct-acting Antiviral Therapies: An International Study
- PMID: 31300820
- PMCID: PMC7245153
- DOI: 10.1093/cid/ciz633
Patterns of Drug and Alcohol Use and Injection Equipment Sharing Among People With Recent Injecting Drug Use or Receiving Opioid Agonist Treatment During and Following Hepatitis C Virus Treatment With Direct-acting Antiviral Therapies: An International Study
Abstract
Background: In many settings, recent or prior injection drug use remains a barrier to accessing direct-acting antiviral treatment (DAA) for hepatitis C virus (HCV) infection. We examined patterns of drug and alcohol use and injection equipment sharing among people with recent injecting drug use or receiving opioid agonist treatment (OAT) during and following DAA-based treatment.
Methods: SIMPLIFY and D3FEAT are phase 4 trials evaluating the efficacy of DAA among people with past 6-month injecting drug use or receiving OAT through a network of 25 international sites. Enrolled in 2016-2017, participants received sofosbuvir/velpatasvir (SIMPLIFY) or paritaprevir/ritonavir/dasabuvir/ombitasvir ± ribavirin (D3FEAT) for 12 weeks and completed behavioral questionnaires before, during, and up to 2 years posttreatment. The impact of time in HCV treatment and follow-up on longitudinally measured longitudinally measured behaviors was estimated using generalized estimating equations.
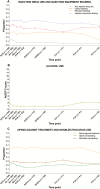
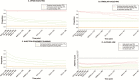
Results: At screening, of 190 participants (mean age, 47 years; 74% male), 62% reported any past-month injecting 16% past-month injection equipment sharing, and 61% current OAT. Median alcohol use was 2 (Alcohol Use Disorders Identification Test-Consumption; range, 1-12). During follow-up, opioid injecting (odds ratio [OR], 0.95; 95% confidence interval [CI], 0.92-0.99) and sharing (OR, 0.87; 95% CI, 0.80-0.94) decreased, whereas no significant changes were observed for stimulant injecting (OR, 0.98; 95% CI, 0.94-1.02) or alcohol use (OR, 0.99; 95% CI, 0.95-1.04).
Conclusions: Injecting drug use and risk behaviors remained stable or decreased following DAA-based HCV treatment. Findings further support expanding HCV treatment to all, irrespective of injection drug use.
Clinical trials registration: SIMPLIFY, NCT02336139; D3FEAT, NCT02498015.
Keywords: DAA; PWID; drug use; hepatitis C; injecting drug use.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2(3): 161–76. - PubMed
-
- World Health Organization. Hepatitis C fact sheet no. 164 Geneva, Switzerland: WHO, 2017.
-
- Larney S, Grebely J, Hickman M, De Angelis D, Dore GJ, Degenhardt L. Defining populations and injecting parameters among people who inject drugs: implications for the assessment of hepatitis C treatment programs. Int J Drug Policy 2015; 26:950–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

