Effects of vagus nerve stimulation are mediated in part by TrkB in a parkinson's disease model
- PMID: 31301412
- PMCID: PMC6701465
- DOI: 10.1016/j.bbr.2019.112080
Effects of vagus nerve stimulation are mediated in part by TrkB in a parkinson's disease model
Abstract
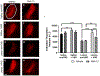
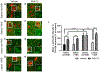
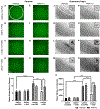
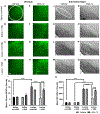
Vagus nerve stimulation (VNS) is being explored as a potential therapeutic for Parkinson's disease (PD). VNS is less invasive than other surgical treatments and has beneficial effects on behavior and brain pathology. It has been suggested that VNS exerts these effects by increasing brain-derived neurotrophic factor (BDNF) to enhance pro-survival mechanisms of its receptor, tropomyosin receptor kinase-B (TrkB). We have previously shown that striatal BDNF is increased after VNS in a lesion model of PD. By chronically administering ANA-12, a TrkB-specific antagonist, we aimed to determine TrkB's role in beneficial VNS effects for a PD model. In this study, we administered a noradrenergic neurotoxin, DSP-4, intraperitoneally and one week later administered a bilateral intrastriatal dopaminergic neurotoxin, 6-OHDA. At this time, the left vagus nerve was cuffed for stimulation. Eleven days later, rats received VNS twice per day for ten days, with daily locomotor assessment. Daily ANA-12 injections were given one hour prior to the afternoon stimulation and concurrent locomotor session. Following the final VNS session, rats were euthanized, and left striatum, bilateral substantia nigra and locus coeruleus were sectioned for immunohistochemical detection of neurons, α-synuclein, astrocytes, and microglia. While ANA-12 did not avert behavioral improvements of VNS, and only partially prevented VNS-induced attenuation of neuronal loss in the locus coeruleus, it did stop neuronal and anti-inflammatory effects of VNS in the nigrostriatal system, indicating a role for TrkB in mediating VNS efficacy. However, our data also suggest that BDNF-TrkB is not the sole mechanism of action for VNS in PD.
Keywords: Brain-derived neurotrophic factor; Dopamine; Norepinephrine; Parkinson’s disease; Tropomyosin receptor kinase B; Vagus nerve stimulation.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures



Similar articles
-
Vagus nerve stimulation improves locomotion and neuronal populations in a model of Parkinson's disease.Brain Stimul. 2017 Nov-Dec;10(6):1045-1054. doi: 10.1016/j.brs.2017.08.008. Epub 2017 Aug 24. Brain Stimul. 2017. PMID: 28918943 Free PMC article.
-
Differential effects of vagus nerve stimulation paradigms guide clinical development for Parkinson's disease.Brain Stimul. 2020 Sep-Oct;13(5):1323-1332. doi: 10.1016/j.brs.2020.06.078. Epub 2020 Jul 3. Brain Stimul. 2020. PMID: 32629028
-
Vagus nerve stimulation with a small total charge transfer improves motor behavior and reduces neuroinflammation in a mouse model of Parkinson's disease.Neurochem Int. 2024 Nov;180:105871. doi: 10.1016/j.neuint.2024.105871. Epub 2024 Oct 1. Neurochem Int. 2024. PMID: 39362497
-
BDNF/TrkB activators in Parkinson's disease: A new therapeutic strategy.J Cell Mol Med. 2024 May;28(10):e18368. doi: 10.1111/jcmm.18368. J Cell Mol Med. 2024. PMID: 38752280 Free PMC article. Review.
-
Vagal Nerve Stimulation for Treatment-Resistant Depression.Neurotherapeutics. 2017 Jul;14(3):716-727. doi: 10.1007/s13311-017-0537-8. Neurotherapeutics. 2017. PMID: 28585221 Free PMC article. Review.
Cited by
-
Right vagus nerve stimulation improves motor behavior by exerting neuroprotective effects in Parkinson's disease rats.Ann Transl Med. 2022 Dec;10(24):1314. doi: 10.21037/atm-22-5366. Ann Transl Med. 2022. PMID: 36660708 Free PMC article.
-
BDNF alleviates Parkinson's disease by promoting STAT3 phosphorylation and regulating neuronal autophagy.Cell Tissue Res. 2023 Sep;393(3):455-470. doi: 10.1007/s00441-023-03806-1. Epub 2023 Jul 14. Cell Tissue Res. 2023. PMID: 37450039 Free PMC article.
-
Effect of Transcutaneous Auricular Vagus Nerve Stimulation on Protracted Alcohol Withdrawal Symptoms in Male Alcohol-Dependent Patients.Front Psychiatry. 2021 Aug 30;12:678594. doi: 10.3389/fpsyt.2021.678594. eCollection 2021. Front Psychiatry. 2021. PMID: 34526917 Free PMC article.
-
Immediate modulatory effects of transcutaneous vagus nerve stimulation on patients with Parkinson's disease: a crossover self-controlled fMRI study.Front Aging Neurosci. 2024 Oct 23;16:1444703. doi: 10.3389/fnagi.2024.1444703. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39507202 Free PMC article.
-
Vagus nerve stimulation in Parkinson's disease: a scoping review of animal studies and human subjects research.NPJ Parkinsons Dis. 2024 Oct 24;10(1):199. doi: 10.1038/s41531-024-00803-1. NPJ Parkinsons Dis. 2024. PMID: 39448636 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

