Differential Patterns of Visual Sensory Alteration Underlying Face Emotion Recognition Impairment and Motion Perception Deficits in Schizophrenia and Autism Spectrum Disorder
- PMID: 31301757
- PMCID: PMC7197738
- DOI: 10.1016/j.biopsych.2019.05.016
Differential Patterns of Visual Sensory Alteration Underlying Face Emotion Recognition Impairment and Motion Perception Deficits in Schizophrenia and Autism Spectrum Disorder
Abstract
Background: Impaired face emotion recognition (FER) and abnormal motion processing are core features in schizophrenia (SZ) and autism spectrum disorder (ASD) that have been linked to atypical activity within the visual cortex. Despite overlaps, only a few studies have directly explored convergent versus divergent neural mechanisms of altered visual processing in ASD and SZ. We employed a multimodal imaging approach to evaluate FER and motion perception in relation to functioning of subcortical and cortical visual regions.
Methods: Subjects were 20 high-functioning adults with ASD, 19 patients with SZ, and 17 control participants. Behavioral measures of coherent motion sensitivity and FER along with electrophysiological and functional magnetic resonance imaging measures of visual pattern and motion processing were obtained. Resting-state functional magnetic resonance imaging was used to assess the relationship between corticocortical and thalamocortical connectivity and atypical visual processing.
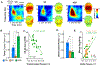
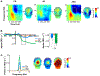
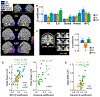
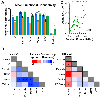
Results: SZ and ASD participants had intercorrelated deficits in FER and motion sensitivity. In both groups, reduced motion sensitivity was associated with reduced functional magnetic resonance imaging activation in the occipitotemporal cortex and lower delta-band electroencephalogram power. In ASD, FER deficits correlated with hyperactivation of dorsal stream regions and increased evoked theta power. Activation of the pulvinar correlated with abnormal alpha-band modulation in SZ and ASD with under- and overmodulation, respectively, predicting increased clinical symptoms in both groups.
Conclusions: SZ and ASD participants showed equivalent deficits in FER and motion sensitivity but markedly different profiles of physiological dysfunction. The specific pattern of deficits observed in each group may help guide development of treatments designed to downregulate versus upregulate visual processing within the respective clinical groups.
Keywords: Autism; EEG; FER; Motion; Schizophrenia; Visual.
Copyright © 2019 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Figures
Comment in
-
Looking Under the Hood of Convergent Behavioral Deficits in Schizophrenia and Autism.Biol Psychiatry. 2019 Oct 1;86(7):e21-e23. doi: 10.1016/j.biopsych.2019.07.030. Biol Psychiatry. 2019. PMID: 31521210 No abstract available.
References
-
- Edwards J, Jackson HJ, Pattison PE (2002): Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 22:789–832. - PubMed
-
- Harms MB, Martin A, Wallace GL (2010): Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychol Rev. 20:290–322. - PubMed
-
- Uljarevic M, Hamilton A (2013): Recognition of emotions in autism: a formal meta-analysis. J Autism Dev Disord. 43:1517–1526. - PubMed

