Evaporative cooling provides a major metabolic energy sink
- PMID: 31302039
- PMCID: PMC6717770
- DOI: 10.1016/j.molmet.2019.06.023
Evaporative cooling provides a major metabolic energy sink
Abstract
Objective: Elimination of food calories as heat could help redress the excess accumulation of metabolic energy exhibited as obesity. Prior studies have focused on the induction of thermogenesis in beige and brown adipose tissues as the application of this principle, particularly because the β-adrenergic environment associated with thermogenic activation has been shown to have positive health implications. The counterpoint to this strategy is the regulation of heat loss; we propose that mammals with inefficient heat conservation will require more thermogenesis to maintain body temperature.
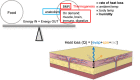
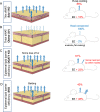
Methods: Surface temperature thermography and rates of trans-epidermal water loss were integrated to profile the total heat transfer of genetically-engineered and genetically variable mice.
Results: These data were incorporated with energy expenditure data to generate a biophysical profile to test the significance of increased rates of evaporative cooling.
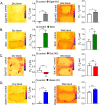
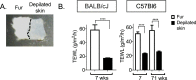
Conclusions: We show that mouse skins vary considerably in their heat retention properties, whether because of naturally occurring variation (SKH-1 mice), or genetic modification of the heat-retaining lipid lamellae (SCD1, DGAT1 or Agouti Ay obese mice). In particular, we turn attention to widely different rates of evaporative cooling as the result of trans-epidermal water loss; higher rates of heat loss by evaporative cooling leads to increased demand for thermogenesis. We speculate that this physiology could be harnessed to create an energy sink to assist with strategies aimed at treating metabolic diseases.
Keywords: Brown adipose tissue; Dermal white adipose tissue; Energy expenditure; Epidermal barrier; Evaporative cooling; Mouse skin; Obesity; Syndecan-1; Thermogenesis; Trans-epidermal water loss.
Copyright © 2019 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures






References
-
- Azzi L., El-Alfy M., Martel C., Labrie F. Gender differences in mouse skin morphology and specific effects of sex steroids and dehydroepiandrosterone. Journal of Investigative Dermatology. 2005;124(1):22–27. - PubMed
-
- Bartelt A., Heeren J. The holy grail of metabolic disease: brown adipose tissue. Current Opinion in Lipidology. 2012;23(3):190–195. - PubMed
-
- Bartelt A., Heeren J. Adipose tissue browning and metabolic health. Nature Reviews Endocrinology. 2014;10(1):24–36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

