VISAGE Reveals a Targetable Mitotic Spindle Vulnerability in Cancer Cells
- PMID: 31302152
- PMCID: PMC6688637
- DOI: 10.1016/j.cels.2019.05.009
VISAGE Reveals a Targetable Mitotic Spindle Vulnerability in Cancer Cells
Abstract
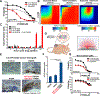
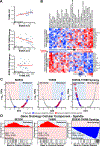
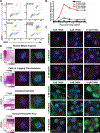
There is an unmet need for new antimitotic drug combinations that target cancer-specific vulnerabilities. Based on our finding of elevated biomolecule oxidation in mitotically arrested cancer cells, we combined Plk1 inhibitors with TH588, an MTH1 inhibitor that prevents detoxification of oxidized nucleotide triphosphates. This combination showed robust synergistic killing of cancer, but not normal, cells that, surprisingly, was MTH1-independent. To dissect the underlying synergistic mechanism, we developed VISAGE, a strategy integrating experimental synergy quantification with computational-pathway-based gene expression analysis. VISAGE predicted, and we experimentally confirmed, that this synergistic combination treatment targeted the mitotic spindle. Specifically, TH588 binding to β-tubulin impaired microtubule assembly, which when combined with Plk1 blockade, synergistically disrupted mitotic chromosome positioning to the spindle midzone. These findings identify a cancer-specific mitotic vulnerability that is targetable using Plk1 inhibitors with microtubule-destabilizing agents and highlight the general utility of the VISAGE approach to elucidate molecular mechanisms of drug synergy.
Keywords: MTH1; Plk1; TH588; anti-microtubule drugs; cancer therapy; chromosome alignment; chromosome congression; drug synergy; mitosis; tubulin.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
Michael B. Yaffe is a member of the Divisions of Surgical Oncology and Trauma and Critical Care, Department of Surgery, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, and the Chief Academic Editor of the journal Science Signaling. The authors declare no competing interests.
Figures




Similar articles
-
The MTH1 inhibitor TH588 is a microtubule-modulating agent that eliminates cancer cells by activating the mitotic surveillance pathway.Sci Rep. 2019 Oct 11;9(1):14667. doi: 10.1038/s41598-019-51205-w. Sci Rep. 2019. PMID: 31604991 Free PMC article.
-
Polo-like kinase 1 inhibitor BI2536 causes mitotic catastrophe following activation of the spindle assembly checkpoint in non-small cell lung cancer cells.Cancer Lett. 2015 Feb 28;357(2):591-601. doi: 10.1016/j.canlet.2014.12.023. Epub 2014 Dec 15. Cancer Lett. 2015. PMID: 25524551
-
Plk1 inhibition leads to a failure of mitotic division during the first mitotic division in pig embryos.J Assist Reprod Genet. 2017 Mar;34(3):399-407. doi: 10.1007/s10815-016-0864-4. Epub 2017 Jan 10. J Assist Reprod Genet. 2017. PMID: 28074435 Free PMC article.
-
Tubulin-associated drug targets: Aurora kinases, Polo-like kinases, and others.Semin Oncol. 2006 Aug;33(4):436-48. doi: 10.1053/j.seminoncol.2006.04.007. Semin Oncol. 2006. PMID: 16890798 Review.
-
Polo-like kinase 1 inhibitors in mono- and combination therapies: a new strategy for treating malignancies.Expert Rev Anticancer Ther. 2011 Jul;11(7):1115-30. doi: 10.1586/era.11.61. Expert Rev Anticancer Ther. 2011. PMID: 21806334 Review.
Cited by
-
Plk1 Inhibitors and Abiraterone Synergistically Disrupt Mitosis and Kill Cancer Cells of Disparate Origin Independently of Androgen Receptor Signaling.Cancer Res. 2023 Jan 18;83(2):219-238. doi: 10.1158/0008-5472.CAN-22-1533. Cancer Res. 2023. PMID: 36413141 Free PMC article.
-
AXL and CAV-1 play a role for MTH1 inhibitor TH1579 sensitivity in cutaneous malignant melanoma.Cell Death Differ. 2020 Jul;27(7):2081-2098. doi: 10.1038/s41418-019-0488-1. Epub 2020 Jan 9. Cell Death Differ. 2020. PMID: 31919461 Free PMC article.
-
Identification of novel microtubule inhibitors effective in fission yeast and human cells and their effects on breast cancer cell lines.Open Biol. 2021 Sep;11(9):210161. doi: 10.1098/rsob.210161. Epub 2021 Sep 8. Open Biol. 2021. PMID: 34493069 Free PMC article.
-
Karonudib has potent anti-tumor effects in preclinical models of B-cell lymphoma.Sci Rep. 2021 Mar 18;11(1):6317. doi: 10.1038/s41598-021-85613-8. Sci Rep. 2021. PMID: 33737576 Free PMC article.
-
MTH1 Inhibitor TH1579 Induces Oxidative DNA Damage and Mitotic Arrest in Acute Myeloid Leukemia.Cancer Res. 2021 Nov 15;81(22):5733-5744. doi: 10.1158/0008-5472.CAN-21-0061. Epub 2021 Sep 30. Cancer Res. 2021. PMID: 34593524 Free PMC article.
References
-
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta crystallographica Section D, Biological crystallography 66, 213–221. - PMC - PubMed
-
- Alexander J, Lim D, Joughin BA, Hegemann B, Hutchins JR, Ehrenberger T, Ivins F, Sessa F, Hudecz O, Nigg EA, et al. (2011). Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling. Sci Signal 4, ra42. - PMC - PubMed
-
- Alexandre J, Batteux F, Nicco C, Chereau C, Laurent A, Guillevin L, Weill B, and Goldwasser F (2006). Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer 119, 41–48. - PubMed
-
- Archambault V, and Glover DM (2009). Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol 10, 265–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

