Epithelial sodium channels in endothelial cells mediate diet-induced endothelium stiffness and impaired vascular relaxation in obese female mice
- PMID: 31302199
- PMCID: PMC6901094
- DOI: 10.1016/j.metabol.2019.153946
Epithelial sodium channels in endothelial cells mediate diet-induced endothelium stiffness and impaired vascular relaxation in obese female mice
Abstract
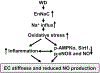
Objective: Mineralocorticoid receptor activation of the epithelial sodium channel in endothelial cells (ECs) (EnNaC) is accompanied by aldosterone induced endothelial stiffening and impaired nitric oxide (NO)-mediated arterial relaxation. Recent data support enhanced activity of the alpha subunit of EnNaC (αEnNaC) mediates this aldosterone induced endothelial stiffening and associated endothelial NO synthase (eNOS) activation. There is mounting evidence that diet induced obesity diminishes expression and activation of AMP-activated protein kinase α (AMPKα), sirtuin 1 (Sirt1), which would be expected to lead to impaired downstream eNOS activation. Thereby, we posited that enhanced EnNaC activation contributes to diet induced obesity related increases in stiffness of the endothelium and diminished NO mediated vascular relaxation by increasing oxidative stress and related inhibition of AMPKα, Sirt1, and associated eNOS inactivation.
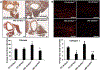
Materials/methods: Sixteen to twenty week-old αEnNaC knockout (αEnNaC-/-) and wild type littermate (EnNaC+/+) female mice were fed a mouse chow or an obesogenic western diet (WD) containing excess fat (46%) and fructose (17.5%) for 16 weeks. Sodium currents of ECs, endothelial stiffness and NO mediated aortic relaxation were examined along with indices of aortic oxidative stress, vascular remodeling and fibrosis.
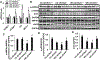
Results: Enhanced EnNaC activation-mediated WD-induced increases in sodium currents in isolated lung ECs, increased endothelial stiffness and impaired aortic endothelium-dependent relaxation to acetylcholine (10-9-10-4 mol/L). These abnormalities occurred in conjunction with WD-mediated aortic tissue oxidative stress, inflammation, and decreased activation of AMPKα, Sirt1, and downstream eNOS were substantially mitigated in αEnNaC-/- mice. Importantly, αEnNaC-/- prevented WD induced increases in endothelial stiffness and related impairment of endothelium-dependent relaxation as well as aortic fibrosis and remodeling. However, EnNaC signaling was not involved in diet-induced abnormal expression of adipokines and CYP11b2 in abdominal aortic perivascular adipose tissue.
Conclusion: These data suggest that endothelial specific EnNaC activation mediates WD-induced endothelial stiffness, impaired eNOS activation, aortic fibrosis and remodeling through increased aortic oxidative stress and increased inflammation related to a reduction of AMPKα and Sirt 1 mediated eNOS phosphorylation/activation and NO production.
Keywords: Endothelial sodium channels; Endothelial stiffness; Fuel sensing kinases; Nitric oxide; Obesity; Oxidative stress.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
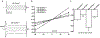
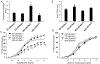
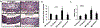
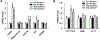
Similar articles
-
Western diet induces renal artery endothelial stiffening that is dependent on the epithelial Na+ channel.Am J Physiol Renal Physiol. 2020 May 1;318(5):F1220-F1228. doi: 10.1152/ajprenal.00517.2019. Epub 2020 Apr 13. Am J Physiol Renal Physiol. 2020. PMID: 32281419 Free PMC article.
-
Epithelial Sodium Channel in Aldosterone-Induced Endothelium Stiffness and Aortic Dysfunction.Hypertension. 2018 Sep;72(3):731-738. doi: 10.1161/HYPERTENSIONAHA.118.11339. Hypertension. 2018. PMID: 29987101 Free PMC article.
-
Endothelial sodium channel activation promotes cardiac stiffness and diastolic dysfunction in Western diet fed female mice.Metabolism. 2020 Aug;109:154223. doi: 10.1016/j.metabol.2020.154223. Epub 2020 Apr 7. Metabolism. 2020. PMID: 32275972 Free PMC article.
-
Role of the vascular endothelial sodium channel activation in the genesis of pathologically increased cardiovascular stiffness.Cardiovasc Res. 2022 Jan 7;118(1):130-140. doi: 10.1093/cvr/cvaa326. Cardiovasc Res. 2022. PMID: 33188592 Free PMC article. Review.
-
Detrimental or beneficial: Role of endothelial ENaC in vascular function.J Cell Physiol. 2022 Jan;237(1):29-48. doi: 10.1002/jcp.30505. Epub 2021 Jul 19. J Cell Physiol. 2022. PMID: 34279047 Review.
Cited by
-
Endothelial sodium channel activation mediates DOCA-salt-induced endothelial cell and arterial stiffening.Metabolism. 2022 May;130:155165. doi: 10.1016/j.metabol.2022.155165. Epub 2022 Feb 17. Metabolism. 2022. PMID: 35183546 Free PMC article.
-
Assessment of iris vasculature in type 2 diabetes mellitus patients without diabetic retinopathy using anterior segment optical coherence tomography angiography.Sci Rep. 2025 May 30;15(1):19035. doi: 10.1038/s41598-025-04463-w. Sci Rep. 2025. PMID: 40447707 Free PMC article.
-
NmFGF1-Regulated Glucolipid Metabolism and Angiogenesis Improves Functional Recovery in a Mouse Model of Diabetic Stroke and Acts via the AMPK Signaling Pathway.Front Pharmacol. 2021 May 7;12:680351. doi: 10.3389/fphar.2021.680351. eCollection 2021. Front Pharmacol. 2021. PMID: 34025437 Free PMC article.
-
Predictive value of estimated pulse wave velocity for cardiovascular and all-cause mortality in individuals with obesity.Diabetol Metab Syndr. 2023 Mar 9;15(1):40. doi: 10.1186/s13098-023-01011-2. Diabetol Metab Syndr. 2023. PMID: 36894988 Free PMC article.
-
Colchicine Alleviates Cholesterol Crystal-Induced Endothelial Cell Pyroptosis through Activating AMPK/SIRT1 Pathway.Oxid Med Cell Longev. 2020 Jul 15;2020:9173530. doi: 10.1155/2020/9173530. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32733639 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

