Development of a multiplex fully automated assay for rapid quantification of CD4+ T cells from whole blood
- PMID: 31302394
- PMCID: PMC6718319
- DOI: 10.1016/j.bios.2019.111490
Development of a multiplex fully automated assay for rapid quantification of CD4+ T cells from whole blood
Abstract
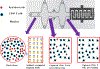
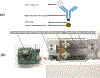
The development of cost-effective and rapid assays for the accurate counting of CD4 cells has remained prime focus for disease management. The lack of such assays has severely affected people living in resource-limited disease prevalent areas. CD4 count information plays a vital role in the effective management of HIV disease. There is an unmet need to develop rapid, cost-effective, portable and user-friendly point-of-care (POC) disease diagnostic platform technology for CD4+ T cell counting. Here, we have developed a flow-free magnetic actuation platform that uses antibody-coated magnetic beads to efficiently capture CD4+ T cells from a 30 μL drop of whole blood. On-chip cell lysate electrical impedance spectroscopy has been utilized to quantify the isolated CD4 cells. The developed assay has a limit of detection of 25 cells per μL and provides accurate CD4 counts in the range of 25-800 cells per μL. The whole immunoassay along with the enumeration process is very rapid and provides CD4 quantification results within 5 min time frame. The assay does not require off-chip sample preparation steps and minimizes human involvement to a greater extent. The developed impedance-based immunoassay has potential to significantly improve the CD4 enumeration process especially for POC settings.
Keywords: Antiretroviral therapy; CD4+ T cells; Human immunodeficiency virus; Rapid immunoassays; Resource-constrained settings.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Rapid, low-cost and instrument-free CD4+ cell counting for HIV diagnostics in resource-poor settings.Lab Chip. 2014 Aug 7;14(15):2844-51. doi: 10.1039/c4lc00264d. Epub 2014 Jun 9. Lab Chip. 2014. PMID: 24911165
-
A low-cost and miniaturized potentiostat for sensing of biomolecular species such as TNF-α by electrochemical impedance spectroscopy.Biosens Bioelectron. 2018 Feb 15;100:533-540. doi: 10.1016/j.bios.2017.09.049. Epub 2017 Sep 28. Biosens Bioelectron. 2018. PMID: 28988118
-
Single wall carbon nanotube electrode system capable of quantitative detection of CD4+ T cells.Biosens Bioelectron. 2017 Apr 15;90:238-244. doi: 10.1016/j.bios.2016.11.055. Epub 2016 Nov 25. Biosens Bioelectron. 2017. PMID: 27914367
-
Microfluidic Devices for HIV Diagnosis and Monitoring at Point-of-Care (POC) Settings.Biosensors (Basel). 2022 Nov 1;12(11):949. doi: 10.3390/bios12110949. Biosensors (Basel). 2022. PMID: 36354458 Free PMC article. Review.
-
Microfluidic Assays for CD4 T Lymphocyte Counting: A Review.Biosensors (Basel). 2025 Jan 9;15(1):33. doi: 10.3390/bios15010033. Biosensors (Basel). 2025. PMID: 39852084 Free PMC article. Review.
Cited by
-
Exosomal biomarkers for cancer diagnosis and patient monitoring.Expert Rev Mol Diagn. 2020 Apr;20(4):387-400. doi: 10.1080/14737159.2020.1731308. Epub 2020 Feb 20. Expert Rev Mol Diagn. 2020. PMID: 32067543 Free PMC article. Review.
-
Microparticle-tagged image-based cell counting (ImmunoSpin) for CD4 + T cells.Mikrochim Acta. 2021 Nov 25;188(12):431. doi: 10.1007/s00604-021-05070-y. Mikrochim Acta. 2021. PMID: 34822013 Free PMC article.
-
RT-LAMP-Based Molecular Diagnostic Set-Up for Rapid Hepatitis C Virus Testing.Biosensors (Basel). 2022 May 5;12(5):298. doi: 10.3390/bios12050298. Biosensors (Basel). 2022. PMID: 35624599 Free PMC article.
-
Nano-engineered screen-printed electrodes: A dynamic tool for detection of viruses.Trends Analyt Chem. 2021 Oct;143:116374. doi: 10.1016/j.trac.2021.116374. Epub 2021 Jun 21. Trends Analyt Chem. 2021. PMID: 34177011 Free PMC article. Review.
-
An inkjet-printed polysaccharide matrix for on-chip sample preparation in point-of-care cell counting chambers.RSC Adv. 2020 May 12;10(31):18062-18072. doi: 10.1039/d0ra01645d. eCollection 2020 May 10. RSC Adv. 2020. PMID: 35517228 Free PMC article.
References
-
- UNAIDS. Global HIV & AIDS statistics — 2018 fact sheet. 2018. [cited 2019 04/01/2019]; Available from: http://www.unaids.org/en/resources/fact-sheet.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

