The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling
- PMID: 31302569
- PMCID: PMC6800624
- DOI: 10.1016/j.coi.2019.05.006
The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling
Abstract
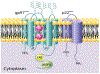
The phagocyte NADPH oxidase possesses a transmembrane electron transferase comprised of gp91phox (aka NOX2) and p22phox and two multicomponent cytosolic complexes, which in stimulated phagocytes translocate to assemble a functional enzyme complex at plasma or phagosomal membranes. The NOX2-centered NADPH oxidase shuttles electrons from cytoplasmic NADPH to molecular oxygen in phagosomes or the extracellular space to produce oxidants that support optimal antimicrobial activity by phagocytes. Additionally, NOX2-generated oxidants have been implicated in both autocrine and paracrine signaling in a variety of biological contexts. However, when interpreting experimental results, investigators must recognize the complexity inherent in the biochemistry of oxidant-mediated attack of microbial targets and the technical limitations of the probes currently used to detect intracellular oxidants.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


References
-
- Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD: Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401:79–82. - PubMed
-
- Banfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, Krause KH: A mammalian H + channel generated through alternative splicing of the NADPH oxidase homolog NOH-1 Science 2000, 287:138–142. - PubMed
-
- Bedard K, Krause KH: The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007, 87:245–313. - PubMed
-
- Lambeth JD, Neish AS: Nox Enzymes and New Thinking on Reactive Oxygen: A Double-Edged Sword Revisited. Annu Rev Pathol 2014, 9:119–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

