Incidence and phenotypes of childhood-onset genetic epilepsies: a prospective population-based national cohort
- PMID: 31302675
- PMCID: PMC6658850
- DOI: 10.1093/brain/awz195
Incidence and phenotypes of childhood-onset genetic epilepsies: a prospective population-based national cohort
Abstract
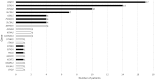
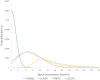
Epilepsy is common in early childhood. In this age group it is associated with high rates of therapy-resistance, and with cognitive, motor, and behavioural comorbidity. A large number of genes, with wide ranging functions, are implicated in its aetiology, especially in those with therapy-resistant seizures. Identifying the more common single-gene epilepsies will aid in targeting resources, the prioritization of diagnostic testing and development of precision therapy. Previous studies of genetic testing in epilepsy have not been prospective and population-based. Therefore, the population-incidence of common genetic epilepsies remains unknown. The objective of this study was to describe the incidence and phenotypic spectrum of the most common single-gene epilepsies in young children, and to calculate what proportion are amenable to precision therapy. This was a prospective national epidemiological cohort study. All children presenting with epilepsy before 36 months of age were eligible. Children presenting with recurrent prolonged (>10 min) febrile seizures; febrile or afebrile status epilepticus (>30 min); or with clusters of two or more febrile or afebrile seizures within a 24-h period were also eligible. Participants were recruited from all 20 regional paediatric departments and four tertiary children's hospitals in Scotland over a 3-year period. DNA samples were tested on a custom-designed 104-gene epilepsy panel. Detailed clinical information was systematically gathered at initial presentation and during follow-up. Clinical and genetic data were reviewed by a multidisciplinary team of clinicians and genetic scientists. The pathogenic significance of the genetic variants was assessed in accordance with the guidelines of UK Association of Clinical Genetic Science (ACGS). Of the 343 patients who met inclusion criteria, 333 completed genetic testing, and 80/333 (24%) had a diagnostic genetic finding. The overall estimated annual incidence of single-gene epilepsies in this well-defined population was 1 per 2120 live births (47.2/100 000; 95% confidence interval 36.9-57.5). PRRT2 was the most common single-gene epilepsy with an incidence of 1 per 9970 live births (10.0/100 000; 95% confidence interval 5.26-14.8) followed by SCN1A: 1 per 12 200 (8.26/100 000; 95% confidence interval 3.93-12.6); KCNQ2: 1 per 17 000 (5.89/100 000; 95% confidence interval 2.24-9.56) and SLC2A1: 1 per 24 300 (4.13/100 000; 95% confidence interval 1.07-7.19). Presentation before the age of 6 months, and presentation with afebrile focal seizures were significantly associated with genetic diagnosis. Single-gene disorders accounted for a quarter of the seizure disorders in this cohort. Genetic testing is recommended to identify children who may benefit from precision treatment and should be mainstream practice in early childhood onset epilepsy.
Keywords: epidemiology; epilepsy; genetics; incidence; precision.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
Comment in
-
Molecular epidemiology of monogenic epilepsies answers key clinical questions.Brain. 2019 Aug 1;142(8):2173-2175. doi: 10.1093/brain/awz208. Brain. 2019. PMID: 31347681 No abstract available.
-
Early-onset genetic epilepsies reaching adult clinics.Brain. 2020 Mar 1;143(3):e19. doi: 10.1093/brain/awaa029. Brain. 2020. PMID: 32203577 Free PMC article. No abstract available.
Similar articles
-
Early childhood epilepsies: epidemiology, classification, aetiology, and socio-economic determinants.Brain. 2021 Oct 22;144(9):2879-2891. doi: 10.1093/brain/awab162. Brain. 2021. PMID: 34687210 Free PMC article.
-
Evaluation of the feasibility, diagnostic yield, and clinical utility of rapid genome sequencing in infantile epilepsy (Gene-STEPS): an international, multicentre, pilot cohort study.Lancet Neurol. 2023 Sep;22(9):812-825. doi: 10.1016/S1474-4422(23)00246-6. Lancet Neurol. 2023. PMID: 37596007 Free PMC article.
-
Genetic and phenotypic heterogeneity suggest therapeutic implications in SCN2A-related disorders.Brain. 2017 May 1;140(5):1316-1336. doi: 10.1093/brain/awx054. Brain. 2017. PMID: 28379373
-
The epidemiology of the epilepsies in children.Ment Retard Dev Disabil Res Rev. 2002;8(3):171-81. doi: 10.1002/mrdd.10035. Ment Retard Dev Disabil Res Rev. 2002. PMID: 12216061 Review.
-
Genetic Landscape of Common Epilepsies: Advancing towards Precision in Treatment.Int J Mol Sci. 2020 Oct 21;21(20):7784. doi: 10.3390/ijms21207784. Int J Mol Sci. 2020. PMID: 33096746 Free PMC article. Review.
Cited by
-
Broadening the scope of multigene panel analysis for adult epilepsy patients.Epilepsia Open. 2024 Aug;9(4):1538-1549. doi: 10.1002/epi4.12993. Epub 2024 Jul 1. Epilepsia Open. 2024. PMID: 38946282 Free PMC article.
-
Efficacy, Tolerability, and Retention of Antiseizure Medications in PRRT2-Associated Infantile Epilepsy.Neurol Genet. 2022 Sep 28;8(5):e200020. doi: 10.1212/NXG.0000000000200020. eCollection 2022 Oct. Neurol Genet. 2022. PMID: 36187725 Free PMC article.
-
Exome sequencing as first-tier genetic testing in infantile-onset pharmacoresistant epilepsy: diagnostic yield and treatment impact.Eur J Hum Genet. 2023 Feb;31(2):179-187. doi: 10.1038/s41431-022-01202-x. Epub 2022 Oct 5. Eur J Hum Genet. 2023. PMID: 36198807 Free PMC article.
-
High-throughput evaluation of epilepsy-associated KCNQ2 variants reveals functional and pharmacological heterogeneity.JCI Insight. 2022 Mar 8;7(5):e156314. doi: 10.1172/jci.insight.156314. JCI Insight. 2022. PMID: 35104249 Free PMC article.
-
Epilepsy-associated SCN2A (Na V 1.2) Variants Exhibit Diverse and Complex Functional Properties.bioRxiv [Preprint]. 2023 Feb 23:2023.02.23.529757. doi: 10.1101/2023.02.23.529757. bioRxiv. 2023. Update in: J Gen Physiol. 2023 Oct 2;155(10):e202313375. doi: 10.1085/jgp.202313375. PMID: 36865317 Free PMC article. Updated. Preprint.
References
-
- Abdelnour E, Gallentine W, McDonald M, Sachdev M, Jiang Y, Mikati MA. Does age affect response to quinidine in patients with KCNT1 mutations? Report of three new cases and review of the literature. Seizure 2018; 55: 1–3. - PubMed
-
- Anagnostou ME, Shiau NY, Taylor RW, McFarland R. Epilepsy due to mutations in the mitochondrial polymerase gamma (POLG) gene: a clinical and molecular genetic review. Epilepsia 2016; 57: 1531–45. - PubMed
-
- Arsov T, Mullen SA, Damiano JA, Lawrence KM, Huh LL, Nolan M et al. . Early-onset absence epilepsy: 1 in 10 cases is caused by GLUT1 deficiency. Epilepsia 2012; 53: e204–7. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

