The relationship between eGFR slope and subsequent risk of vascular outcomes and all-cause mortality in type 2 diabetes: the ADVANCE-ON study
- PMID: 31302707
- PMCID: PMC6805825
- DOI: 10.1007/s00125-019-4948-4
The relationship between eGFR slope and subsequent risk of vascular outcomes and all-cause mortality in type 2 diabetes: the ADVANCE-ON study
Abstract
Aims/hypothesis: Some studies have reported that annual change in eGFR (eGFR slope) is associated with the future risk of end-stage kidney disease, cardiovascular disease and death in general or chronic kidney disease cohorts. However, the benefits of using eGFR slopes for prediction of major clinical outcomes in diabetes are unclear.
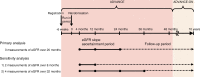
Methods: We used data from the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial and the ADVANCE Post-Trial Observational Study (ADVANCE-ON). After excluding the first 4 months during which an acute fall in eGFR was induced by the initiation of an ACE inhibitor and diuretic combination agent, eGFR slopes were estimated by linear mixed models, using three measurements of eGFR at 4, 12 and 24 months after randomisation over 20 months, and categorised according to quartiles. Cox regression models were used to evaluate adjusted HRs for the study's primary outcome, a composite of major renal events, major macrovascular events and all-cause mortality during the subsequent follow-up from 24 months after randomisation.
Results: A total of 8,879 participants (80%) were included in this cohort. The mean age was 65.6 years (SD 6.3), the mean eGFR was 75 ml min-1 (1.73 m)-2 (SD 17) and the median urinary albumin/creatinine ratio was 14 μg/mg (interquartile range 7-38). The mean eGFR slope was -0.63 ml min-1 (1.73 m)-2 year-1 (SD 1.75). Over a median follow-up of 7.6 years following the 20-month eGFR slope ascertainment period, 2,221 participants (25%) met the primary outcome. An annual substantial decrease in eGFR (lowest 25%, <-1.63 ml min-1 [1.73 m]-2 year-1) was significantly associated with the subsequent risk of the primary outcome (HR 1.30 [95% CI 1.17, 1.43]) compared with a stable change in eGFR (middle 50%, -1.63 to 0.33). An annual substantial increase in eGFR (highest 25%, >0.33) had no significant association with the risk of the primary outcome (HR 0.96 [95% CI 0.86, 1.07]).
Conclusions/interpretation: Our study supports the utility of eGFR slope in type 2 diabetes as a surrogate endpoint for renal outcomes, as well as a prognostic factor for identifying individuals at high risk of cardiovascular disease and all-cause mortality.
Trial registry number: ClinicalTrials.gov registration no. NCT00145925 and no. NCT00949286.
Keywords: Cardiovascular disease; End-stage kidney disease; Mortality; Surrogate endpoint; Type 2 diabetes; eGFR slope.
Conflict of interest statement
MO, TO, TT and TW report no conflicts of interest. MJ reports receiving grant support from the NHMRC of Australia (Project Grant: 1148060) and unrestricted grant support from VentureWise (a wholly owned commercial subsidiary of NPS MedicineWise) to conduct a commissioned project funded by AstraZeneca. MEC received consulting fees from Merck, GlaxoSmithKline, Amgen and AstraZeneca, and lecture fees from Servier. SaH reports personal fees and non-financial support from AstraZeneca, Bristol-Myers Squibb, Janssen, MSD and Sanofi, and personal fees from Abbott, Boehringer Ingelheim, Eli Lilly and Company, Novartis, Novo Nordisk, Servier and Takeda. PH received consulting fees from Servier. StH reports lecture fees from Servier, Takeda and Novartis. GM reports personal fees from Servier, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, Novartis, Menarini Group, Recordati and Takeda Pharmaceutical Company. MM received personal fees from Novo Nordisk, Sanofi, Eli Lilly and Company, Merck Sharp & Dohme, Abbott, Novartis, Servier and AstraZeneca, and grant support from Novo Nordisk, Sanofi, Eli Lilly and Company, Merck Sharp & Dohme and Novartis. BW received lecture fees from Servier, Novartis, Daiichi Sankyo, Pfizer and Boehringer Ingelheim, and serves on trial steering committees for Novartis, Relypsa and Vascular Dynamics. JC received research grants from the NHMRC of Australia and from Servier for the ADVANCE trial and ADVANCE-ON post-trial follow-up, and honoraria for speaking about these studies at scientific meetings. MW reports consultancy fees from Amgen and Kirin and grant support from the NHMRC. VP reports honoraria for scientific lectures from Boehringer Ingelheim, Merck, AbbVie, Roche, AstraZeneca and Servier, and serves on steering committees and advisory boards supported by AbbVie, Astellas, Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Janssen and Pfizer.
Figures


References
-
- Australian Institute of Health and Welfare (2018) Deaths from diabetes. Available from https://www.aihw.gov.au/reports/diabetes/diabetes-snapshot/contents/deat.... Accessed 15 Oct 2018
-
- The United States Renal Data System . 2017 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2017.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

