Oncogene Amplification in Growth Factor Signaling Pathways Renders Cancers Dependent on Membrane Lipid Remodeling
- PMID: 31303424
- PMCID: PMC6742496
- DOI: 10.1016/j.cmet.2019.06.014
Oncogene Amplification in Growth Factor Signaling Pathways Renders Cancers Dependent on Membrane Lipid Remodeling
Abstract
Advances in DNA sequencing technologies have reshaped our understanding of the molecular basis of cancer, providing a precise genomic view of tumors. Complementary biochemical and biophysical perspectives of cancer point toward profound shifts in nutrient uptake and utilization that propel tumor growth and major changes in the structure of the plasma membrane of tumor cells. The molecular mechanisms that bridge these fundamental aspects of tumor biology remain poorly understood. Here, we show that the lysophosphatidylcholine acyltransferase LPCAT1 functionally links specific genetic alterations in cancer with aberrant metabolism and plasma membrane remodeling to drive tumor growth. Growth factor receptor-driven cancers are found to depend on LPCAT1 to shape plasma membrane composition through enhanced saturated phosphatidylcholine content that is, in turn, required for the transduction of oncogenic signals. These results point to a genotype-informed strategy that prioritizes lipid remodeling pathways as therapeutic targets for diverse cancers.
Keywords: cancer dependency; cancer metabolism; gene amplification; growth factor signaling; membrane lipid remodeling.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
PSM is co-founder of Pretzel Therapeutics, Inc. He has equity and serves as a consultant for the company. PSM also did a one-time consultation for Abide Therapeutics, Inc.
Figures


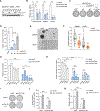


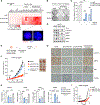

Comment in
-
Tying lipid rafts to oncogenic signalling.Nat Rev Mol Cell Biol. 2019 Sep;20(9):513. doi: 10.1038/s41580-019-0160-0. Nat Rev Mol Cell Biol. 2019. PMID: 31324870 No abstract available.
-
Membrane Lipid Remodeling Takes Center Stage in Growth Factor Receptor-Driven Cancer Development.Cell Metab. 2019 Sep 3;30(3):407-408. doi: 10.1016/j.cmet.2019.08.016. Cell Metab. 2019. PMID: 31484051
References
-
- Abdelzaher E, and Mostafa MF (2015). Lysophosphatidylcholine acyltransferase 1 (LPCAT1) upregulation in breast carcinoma contributes to tumor progression and predicts early tumor recurrence. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 36, 5473–5483. - PubMed
-
- Abercrombie M, and Ambrose EJ (1962). The surface properties of cancer cells: a review. Cancer research 22, 525–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

