Improved synthesis of icosahedral carboranes containing exopolyhedral B-C and C-C bonds
- PMID: 31303685
- PMCID: PMC6625786
- DOI: 10.1016/j.tet.2018.11.040
Improved synthesis of icosahedral carboranes containing exopolyhedral B-C and C-C bonds
Abstract
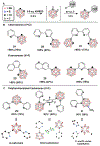
Carboranes are boron-rich molecular clusters possessing electronic characteristics that allow for orthogonal approaches to vertex-selective modifications. We report improved functionalization methods utilizing orthogonal chemistry to achieve efficient substitution at electron-rich B-vertices and electron-poor C-vertices of carborane. Functionalization of B-vertices with alkyl and (hetero)aryl groups using the corresponding Grignard reagents has been improved through the use of a Pd-based precatalyst featuring an electron-rich biaryl phosphine ligand, resulting in reduced reaction times. Importantly, this method is tolerant towards alkyl-based Grignard reagents containing β-hydrogens. Furthermore, a transition metal-free approach to the substitution of carborane C-vertices with (hetero)aryl substrates has been developed under nucleophilic aromatic substitution (SNAr) conditions. The selective substitution of carboranes afforded by these methods holds potential for the rational synthesis of heterofunctionalized boron clusters with substituents on both boron and carbon-based vertices.
Keywords: boron clusters; carborane; catalysis; cross-coupling; nucleophilic aromatic substitution.
Figures
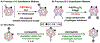


References
-
- Paxton RJ; Beatty BG; Hawthorne MF; Varadarajan A; Williams LE; Curtis FL; Knobler CB; Beatty JD; Shively JE Pro. Natl. Acad. Sci 1991, 88 (8), 3387 - PMC - PubMed
- Hawthorne MF; Maderna A Chem. Rev 1999, 99 (12), 3421. - PubMed
- Neirynck P; Schimer J; Jonkheijm P; Milroy LG; Cigler P; Brunsveld LJ Mater. Chem. B 2015, 3 (4), 539. - PubMed
-
- McArthur SG; Geng L; Guo J; Lavallo V Inorg. Chem. Front 2015, 2 (12), 1101.
-
- Selg C; Neumann W; Lönnecke P; Hey-Hawkins E; Zeitler K Chem. Eur. J 2017, 23 (13), 7932. - PubMed
-
- Farha OK; Spokoyny AM; Mulfort KL; Hawthorne MF; Mirkin CA; Hupp JT J. Am. Chem. Soc 2007, 129 (42), 12680. - PubMed
- Serino AC; Anderson ME; Saleh LMA; Dziedzic RM; Mills H; Heindreich LK; Spokoyny AM; Weiss PS ACS Appl. Mater. Interfaces 2017, 9, 40, 34592. - PubMed
- Kim J; Rim YS; Liu Y; Serino AC; Thomas JC; Chen H; Yang Y; Weiss PS Nano Lett. 2014, 14 (5), 2946. - PubMed
- Spokoyny AM; Farha OK; Mulfort KL; Hupp JT; Mirkin CA Inorg. Chim. Acta 2010, 364 (1), 266.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
