Is there an interaction between dexamethasone and sugammadex in real clinical conditions? A randomized controlled trial in patients undergoing laparoscopic cholecystectomy
- PMID: 31303711
- PMCID: PMC6598587
- DOI: 10.4103/joacp.JOACP_42_17
Is there an interaction between dexamethasone and sugammadex in real clinical conditions? A randomized controlled trial in patients undergoing laparoscopic cholecystectomy
Abstract
Background and aims: There is evidence that sugammadex can encapsulate other substances except rocuronium, such as dexamethasone. The aim of this study was to investigate the possible clinical interaction between dexamethasone and sugammadex, in patients undergoing laparoscopic cholecystectomy.
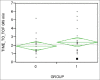
Material and methods: This was a randomized, double-blind controlled trial, performed in patients aged 18-75 years, American Society of Anesthesiologists (ASA) I-III, who underwent a laparoscopic cholecystectomy under deep neuromuscular blockade with rocuronium. Patients received 5 mg of dexamethasone or placebo (N/S 0.9%) during induction of anesthesia. Sugammadex 4 mg/kg was administered at the end of surgery at post-tetanic count 1-2. The outcome measures assessed were the time from sugammadex administration until train-of-four (TOF) 0.9, and until patient's extubation, postoperative pain (measured by numeric rating scale 0-10), nausea and vomiting, as well as rescue analgesics and antiemetics required during the first 24 hours postoperatively. The total dose of rocuronium required in both groups was also recorded.
Results: Overall, 44 patients were studied. No difference was detected regarding the demographic and surgical characteristics of patients. The time from sugammadex administration until TOF 0.9 and until patients' extubation did not differ significantly between the groups (P = 0.21 and 0.17). Operating conditions, pain scores, nausea/vomiting, and rescue analgesics and antiemetics during the first 24 hours postoperatively, did not differ between the groups. The total dose of rocuronium, however, was significantly more in patients who received dexamethasone (P = 0.01).
Conclusion: No significant clinical interaction was revealed between dexamethasone and sugammadex during reversal of deep neuromuscular blockade in patients undergoing laparoscopic cholecystectomy.
Keywords: Dexamethasone; pain; postoperative; sugammadex.
Conflict of interest statement
There are no conflicts of interest.
Figures

References
-
- Kopman AF. Neuromuscular monitoring: Old issues, new controversies. J Crit Care. 2009;24:11–20. - PubMed
-
- Fink H, Hollmann MW. Myths and facts in neuromuscular pharmacology. New developments in reversing neuromuscular blockade. Minerva Anestesiol. 2012;78:473–82. - PubMed
-
- Schaller S, Fink H. A randomized controlled trial comparing sugammadex and neostigmine at different depths of neuromuscular blockade in patients undergoing laparsoscopic surgery. Anaesthesia. 2012;67:991–8. - PubMed
-
- Plaud B, Meretoja O, Hofmockel R, Raft J, Stoddart PA, van Kuijk JH, et al. Reversal of rocuronium-induced neuromuscular blockade with sugammadex in pediatric and adult surgical patients. Anesthesiology. 2009;110:284–94. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

