AGR2, a unique tumor-associated antigen, is a promising candidate for antibody targeting
- PMID: 31303962
- PMCID: PMC6611513
- DOI: 10.18632/oncotarget.26945
AGR2, a unique tumor-associated antigen, is a promising candidate for antibody targeting
Abstract
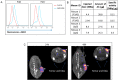
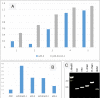
Anterior gradient 2 (AGR2), a protein disulfide isomerase, shows two subcellular localizations: intracellular (iAGR2) and extracellular (eAGR2). In healthy cells that express AGR2, the predominant form is iAGR2, which resides in the endoplasmic reticulum. In contrast, cancer cells secrete and express eAGR2 on the cell surface. We wanted to test if AGR2 is a cancer-specific tumor-associated antigen. We utilized two AGR2 antibodies, P3A5 and P1G4, for in vivo tumor localization and tumor growth inhibition. The monoclonal antibodies recognized both human AGR2 and mouse Agr2. Biodistribution experiments using a syngeneic mouse model showed high uptake of P3A5 AGR2 antibody in xenografted eAgr2+ pancreatic tumors, with limited uptake in normal tissues. In implanted human patient-derived eAGR2+ pancreatic cancer xenografts, tumor growth inhibition was evaluated with antibodies and Gemcitabine (Gem). Inhibition was more potent by P1G4 + Gem combination than Gem alone or P3A5 + Gem. We converted these two antibodies to human:mouse chimeric forms: the constructed P3A5 and P1G4 chimeric mVLhCκ and mVHhCγ (γ1, γ2, γ4) genes were inserted in a single mammalian expression plasmid vector, and transfected into human 293F cells. Expressed human:mouse chimeric IgG1, IgG2 and IgG4 antibodies retained AGR2 binding. Increase in IgG yield by transfected cells could be obtained with serial transfection of vectors with different drug resistance. These chimeric antibodies, when incubated with human blood, effectively lysed eAGR2+ PC3 prostate cancer cells. We have, thus, produced humanized anti-AGR2 antibodies that, after further testing, might be suitable for treatment against a variety of eAGR2+ solid tumors.
Keywords: chimeric antibody; eAGR2; pancreatic cancer; prostate cancer; tumor localization.
Conflict of interest statement
CONFLICTS OF INTEREST The authors do not have any conflicts of interest.
Figures




References
-
- Pascal LE, Vêncio RZ, Page LS, Liebeskind ES, Shadle CP, Troisch P, Marzolf B, True LD, Hood LE, Liu AY. Gene expression relationship between prostate cancer cells of Gleason 3, 4 and normal epithelial cells as revealed by cell type-specific transcriptomes. BMC Cancer. 2009; 9:452. 10.1186/1471-2407-9-452. . - DOI - PMC - PubMed
-
- Dumartin L, Whiteman HJ, Weeks ME, Hariharan D, Dmitrovic B, Iacobuzio-Donahue CA, Brentnall TA, Bronner MP, Feakins RM, Timms JF, Brennan C, Lemoine NR, Crnogorac-Jurcevic T. AGR2 is a novel surface antigen that promotes the dissemination of pancreatic cancer cells through regulation of cathepsins B and D. Cancer Res. 2011; 71:7091-7102. 10.1158/0008-5472.CAN-11-1367. . - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

