Recent advances in the genome mining of Aspergillus secondary metabolites (covering 2012-2018)
- PMID: 31303983
- PMCID: PMC6590338
- DOI: 10.1039/c9md00054b
Recent advances in the genome mining of Aspergillus secondary metabolites (covering 2012-2018)
Abstract
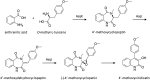
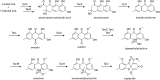
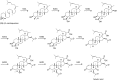
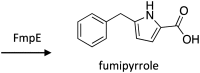
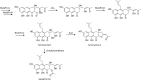
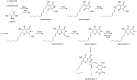
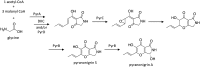
Secondary metabolites (SMs) produced by filamentous fungi possess diverse bioactivities that make them excellent drug candidates. Whole genome sequencing has revealed that fungi have the capacity to produce a far greater number of SMs than have been isolated, since many of the genes involved in SM biosynthesis are either silent or expressed at very low levels in standard laboratory conditions. There has been significant effort to activate SM biosynthetic genes and link them to their downstream products, as the SMs produced by these "cryptic" pathways offer a promising source for new drug discovery. Further, an understanding of the genes involved in SM biosynthesis facilitates product yield optimization of first-generation molecules and genetic engineering of second-generation analogs. This review covers advances made in genome mining SMs produced by Aspergillus nidulans, Aspergillus fumigatus, Aspergillus niger, and Aspergillus terreus in the past six years (2012-2018). Genetic identification and molecular characterization of SM biosynthetic gene clusters, along with proposed biosynthetic pathways, will be discussed in depth.
Figures



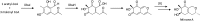
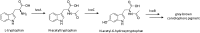


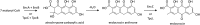

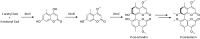


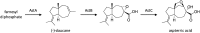
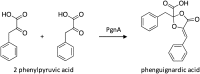
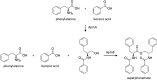
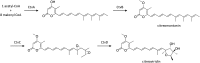


Similar articles
-
Recent advances in genome mining of secondary metabolites in Aspergillus terreus.Front Microbiol. 2014 Dec 23;5:717. doi: 10.3389/fmicb.2014.00717. eCollection 2014. Front Microbiol. 2014. PMID: 25566227 Free PMC article. Review.
-
Recent advances in genome mining of secondary metabolite biosynthetic gene clusters and the development of heterologous expression systems in Aspergillus nidulans.J Ind Microbiol Biotechnol. 2014 Feb;41(2):433-42. doi: 10.1007/s10295-013-1386-z. Epub 2013 Dec 17. J Ind Microbiol Biotechnol. 2014. PMID: 24342965 Free PMC article. Review.
-
Beyond the Biosynthetic Gene Cluster Paradigm: Genome-Wide Coexpression Networks Connect Clustered and Unclustered Transcription Factors to Secondary Metabolic Pathways.Microbiol Spectr. 2021 Oct 31;9(2):e0089821. doi: 10.1128/Spectrum.00898-21. Epub 2021 Sep 15. Microbiol Spectr. 2021. PMID: 34523946 Free PMC article.
-
A genomics based discovery of secondary metabolite biosynthetic gene clusters in Aspergillus ustus.PLoS One. 2015 Feb 23;10(2):e0116089. doi: 10.1371/journal.pone.0116089. eCollection 2015. PLoS One. 2015. PMID: 25706180 Free PMC article.
-
An efficient system for heterologous expression of secondary metabolite genes in Aspergillus nidulans.J Am Chem Soc. 2013 May 22;135(20):7720-31. doi: 10.1021/ja401945a. Epub 2013 May 9. J Am Chem Soc. 2013. PMID: 23621425 Free PMC article.
Cited by
-
Biosynthesis of Antibacterial Iron-Chelating Tropolones in Aspergillus nidulans as Response to Glycopeptide-Producing Streptomycetes.Front Fungal Biol. 2022 Jan 3;2:777474. doi: 10.3389/ffunb.2021.777474. eCollection 2021. Front Fungal Biol. 2022. PMID: 37744088 Free PMC article.
-
Metabolomic Analysis of Aspergillus niger Isolated From the International Space Station Reveals Enhanced Production Levels of the Antioxidant Pyranonigrin A.Front Microbiol. 2020 May 21;11:931. doi: 10.3389/fmicb.2020.00931. eCollection 2020. Front Microbiol. 2020. PMID: 32670208 Free PMC article.
-
Regulation of pseurotin A biosynthesis by GliZ and zinc in Aspergillus fumigatus.Sci Rep. 2023 Feb 10;13(1):2431. doi: 10.1038/s41598-023-29753-z. Sci Rep. 2023. PMID: 36765124 Free PMC article.
-
Absolute Stereochemistry and Cytotoxic Effects of Vismione E from Marine Sponge-Derived Fungus Aspergillus sp. 1901NT-1.2.2.Int J Mol Sci. 2023 May 2;24(9):8150. doi: 10.3390/ijms24098150. Int J Mol Sci. 2023. PMID: 37175852 Free PMC article.
-
Discovery and Functional Analysis of a Salicylic Acid Hydroxylase from Aspergillus niger.Appl Environ Microbiol. 2021 Feb 26;87(6):e02701-20. doi: 10.1128/AEM.02701-20. Print 2021 Feb 26. Appl Environ Microbiol. 2021. PMID: 33397706 Free PMC article.
References
-
- Demain A. L. and Fang A., in History of Modern Biotechnology I, Springer, Berlin, Heidelberg, 2000, pp. 1–39.
-
- Fischbach M. A., Walsh C. T. Chem. Rev. 2006;106:3468–3496. - PubMed
-
- Flores-Bustamante Z. R., Rivera-Orduña F. N., Martínez-Cárdenas A., Flores-Cotera L. B. J. Antibiot. 2010;63:460–467. - PubMed
-
- Colombo D., Ammirati E. J. Biol. Regul. Homeostatic Agents. 2011;25:493–504. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

