Revitalizing antifolates through understanding mechanisms that govern susceptibility and resistance
- PMID: 31303985
- PMCID: PMC6595967
- DOI: 10.1039/c9md00078j
Revitalizing antifolates through understanding mechanisms that govern susceptibility and resistance
Abstract
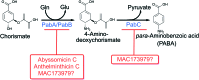
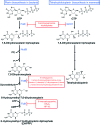
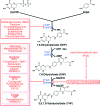
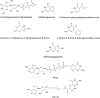
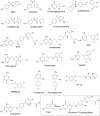
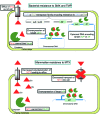
In prokaryotes and eukaryotes, folate (vitamin B9) is an essential metabolic cofactor required for all actively growing cells. Specifically, folate serves as a one-carbon carrier in the synthesis of amino acids (such as methionine, serine, and glycine), N-formylmethionyl-tRNA, coenzyme A, purines and thymidine. Many microbes are unable to acquire folates from their environment and rely on de novo folate biosynthesis. In contrast, mammals lack the de novo folate biosynthesis pathway and must obtain folate from commensal microbiota or the environment using proton-coupled folate transporters. The essentiality and dichotomy between mammalian and bacterial folate biosynthesis and utilization pathways make it an ideal drug target for the development of antimicrobial agents and cancer chemotherapeutics. In this minireview, we discuss general aspects of folate biosynthesis and the underlying mechanisms that govern susceptibility and resistance of organisms to antifolate drugs.
Figures

Similar articles
-
Molecular basis of antifolate resistance.Cancer Metastasis Rev. 2007 Mar;26(1):153-81. doi: 10.1007/s10555-007-9049-z. Cancer Metastasis Rev. 2007. PMID: 17333344 Review.
-
The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis.Drug Resist Updat. 2006 Aug-Oct;9(4-5):227-46. doi: 10.1016/j.drup.2006.09.001. Epub 2006 Nov 7. Drug Resist Updat. 2006. PMID: 17092765 Review.
-
Folylpoly-γ-glutamate synthetase: A key determinant of folate homeostasis and antifolate resistance in cancer.Drug Resist Updat. 2016 Sep;28:43-64. doi: 10.1016/j.drup.2016.06.004. Epub 2016 Jul 4. Drug Resist Updat. 2016. PMID: 27620954 Review.
-
Anticancer antifolates: current status and future directions.Curr Pharm Des. 2003;9(31):2593-613. doi: 10.2174/1381612033453712. Curr Pharm Des. 2003. PMID: 14529544 Review.
-
Molecular and cellular biology of the human reduced folate carrier.Prog Nucleic Acid Res Mol Biol. 2001;67:131-62. doi: 10.1016/s0079-6603(01)67027-2. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11525381 Review.
Cited by
-
An improved statistical method to identify chemical-genetic interactions by exploiting concentration-dependence.PLoS One. 2021 Oct 1;16(10):e0257911. doi: 10.1371/journal.pone.0257911. eCollection 2021. PLoS One. 2021. PMID: 34597304 Free PMC article.
-
Syntheses and biological activities of calix[4]resorcinarene derivatives modified by sulfonic acid and sulfonamides.RSC Adv. 2024 Aug 12;14(35):25115-25119. doi: 10.1039/d4ra04426f. eCollection 2024 Aug 12. RSC Adv. 2024. PMID: 39139234 Free PMC article.
-
Transcript Profiling of Nitroxoline-Treated Biofilms Shows Rapid Up-regulation of Iron Acquisition Gene Clusters.ACS Infect Dis. 2022 Aug 12;8(8):1594-1605. doi: 10.1021/acsinfecdis.2c00206. Epub 2022 Jul 13. ACS Infect Dis. 2022. PMID: 35830188 Free PMC article.
-
Microbial Resistance to Antibiotics and Effective Antibiotherapy.Biomedicines. 2022 May 12;10(5):1121. doi: 10.3390/biomedicines10051121. Biomedicines. 2022. PMID: 35625857 Free PMC article. Review.
-
Identification and characterization of archaeal pseudomurein biosynthesis genes through pangenomics.mSystems. 2025 Mar 18;10(3):e0140124. doi: 10.1128/msystems.01401-24. Epub 2025 Feb 12. mSystems. 2025. PMID: 39936904 Free PMC article.
References
-
- Wen X., Wang J.-S., Backman J. T., Laitila J., Neuvonen P. J. Drug Metab. Dispos. 2002;30:631–635. - PubMed
-
- Strevel E. L., Kuper A., Gold W. L. Lancet Infect. Dis. 2006;6:178–182. - PubMed
-
- Keisu M., Wiholm B.-E., Palmblad J. J. Intern. Med. 1990;228:353–360. - PubMed
-
- Smilack J. Mayo Clin. Proc. 1999;74:730–734. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

