Rational design of novel amphipathic antimicrobial peptides focused on the distribution of cationic amino acid residues
- PMID: 31303986
- PMCID: PMC6590335
- DOI: 10.1039/c9md00166b
Rational design of novel amphipathic antimicrobial peptides focused on the distribution of cationic amino acid residues
Abstract
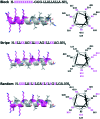
Antimicrobial peptides (AMPs) have garnered much attention as novel therapeutic agents against infectious diseases. They exhibit antimicrobial activity through microbial membrane disruption based on their amphipathic properties. In this study, we rationally designed and synthesized a series of novel AMPs Block, Stripe, and Random, and revealed that Stripe exhibits potent antimicrobial activity against Gram-positive and Gram-negative microbes. Moreover, we also demonstrated that Stripe disrupts both Gram-positive and Gram-negative mimetic bacterial membranes. Finally, we investigated the hemolytic activity and cytotoxicity in human blood cells and human cell lines, and found that Stripe exhibited neither. These data indicated that Stripe is a promising antimicrobial reagent that does not display significant cytotoxicity.
Figures



Similar articles
-
Structure-activity relationship study of amphipathic antimicrobial peptides using helix-destabilizing sarcosine.J Pept Sci. 2021 Dec;27(12):e3360. doi: 10.1002/psc.3360. Epub 2021 Jun 23. J Pept Sci. 2021. PMID: 34164880
-
Rational Design of Helix-Stabilized Antimicrobial Peptide Foldamers Containing α,α-Disubstituted Amino Acids or Side-Chain Stapling.Chempluschem. 2020 Dec;85(12):2731-2736. doi: 10.1002/cplu.202000749. Chempluschem. 2020. PMID: 33369262
-
Rational Design of Amphipathic Antimicrobial Peptides with Alternating L-/D-Amino Acids That Form Helical Structures.Chem Pharm Bull (Tokyo). 2024;72(2):149-154. doi: 10.1248/cpb.c23-00465. Chem Pharm Bull (Tokyo). 2024. PMID: 38296556
-
Antimicrobial peptides: promising compounds against pathogenic microorganisms.Curr Med Chem. 2014;21(20):2299-321. doi: 10.2174/0929867321666140217110155. Curr Med Chem. 2014. PMID: 24533812 Review.
-
Defensive remodeling: How bacterial surface properties and biofilm formation promote resistance to antimicrobial peptides.Biochim Biophys Acta. 2015 Nov;1848(11 Pt B):3089-100. doi: 10.1016/j.bbamem.2015.05.022. Epub 2015 Jun 4. Biochim Biophys Acta. 2015. PMID: 26051126 Review.
Cited by
-
The effects of magainin 2-derived and rationally designed antimicrobial peptides on Mycoplasma pneumoniae.PLoS One. 2022 Jan 24;17(1):e0261893. doi: 10.1371/journal.pone.0261893. eCollection 2022. PLoS One. 2022. PMID: 35073323 Free PMC article.
-
Synergy between the clavanins as a weapon against multidrug-resistant Enterobacter cloacae.RSC Med Chem. 2024 May 16;15(6):2160-2164. doi: 10.1039/d4md00070f. eCollection 2024 Jun 19. RSC Med Chem. 2024. PMID: 38911167 Free PMC article.
-
Development of Antimicrobial Stapled Peptides Based on Magainin 2 Sequence.Molecules. 2021 Jan 16;26(2):444. doi: 10.3390/molecules26020444. Molecules. 2021. PMID: 33466998 Free PMC article.
-
Enhancing Chemical Stability through Structural Modification of Antimicrobial Peptides with Non-Proteinogenic Amino Acids.Antibiotics (Basel). 2023 Aug 17;12(8):1326. doi: 10.3390/antibiotics12081326. Antibiotics (Basel). 2023. PMID: 37627746 Free PMC article.
-
Rational Design of Antimicrobial Peptides Based on Bacterial Type‑I Toxins AapA1, IbsC, and Fst1.ACS Omega. 2025 Jun 9;10(24):25790-25800. doi: 10.1021/acsomega.5c01863. eCollection 2025 Jun 24. ACS Omega. 2025. PMID: 40584331 Free PMC article.
References
-
- Song J. H. Int. J. Infect. Dis. 2003;1:S1–S4. - PubMed
- Suga T., Yamaguchi K. Asian Med. J. 2009;52:103.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

