Stereoselective semi-synthesis of the neuroprotective natural product, serofendic acid
- PMID: 31303993
- PMCID: PMC6595966
- DOI: 10.1039/c9md00145j
Stereoselective semi-synthesis of the neuroprotective natural product, serofendic acid
Abstract
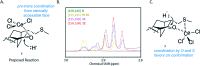
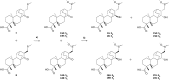
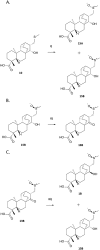
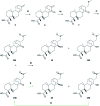
We have recently demonstrated a synthetic biology-enabled semi-synthesis of the potent neuroprotective compound, serofendic acid. An engineered bacterium produces ent-atis-16-en-19-oic acid, which has six of eight chiral carbons configured with the appropriate stereochemistry. Setting the configuration of the C15 hydroxyl group and C16 methylene is a critical step that occurs late in each published total or formal synthesis. Here we explore the use of alternative reducing reagents, stereochemical directing agents, reaction order, and product recycling to improve the diastereoselectivity of this step. We find that installing and oxidizing the C17 methylsulfide prior to reducing the C15 ketone provides the greatest yield of the desired C15,C16 diastereomer. This represents an improved total synthesis of serofendic acid.
Figures

Similar articles
-
Pharmacological and physiological properties of serofendic acid, a novel neuroprotective substance isolated from fetal calf serum.Life Sci. 2003 Dec 5;74(2-3):263-9. doi: 10.1016/j.lfs.2003.09.013. Life Sci. 2003. PMID: 14607254 Review.
-
Semisynthesis of the Neuroprotective Metabolite, Serofendic Acid.ACS Synth Biol. 2019 Oct 18;8(10):2397-2403. doi: 10.1021/acssynbio.9b00261. Epub 2019 Sep 17. ACS Synth Biol. 2019. PMID: 31487457
-
A synthesis-enabled relative stereochemical assignment of the C1-C28 region of hemicalide.Chem Commun (Camb). 2018 Mar 27;54(26):3247-3250. doi: 10.1039/c8cc00933c. Chem Commun (Camb). 2018. PMID: 29536067
-
Isolation of a diterpenoid substance with potent neuroprotective activity from fetal calf serum.Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):3288-93. doi: 10.1073/pnas.052693999. Epub 2002 Feb 26. Proc Natl Acad Sci U S A. 2002. PMID: 11867740 Free PMC article.
-
[Endogenous factors regulating neuronal apoptosis].Nihon Shinkei Seishin Yakurigaku Zasshi. 2005 Oct;25(5):245-50. Nihon Shinkei Seishin Yakurigaku Zasshi. 2005. PMID: 16313102 Review. Japanese.
References
-
- Gooch C. L., Pracht E., Borenstein A. R. Ann. Neurol. 2017;81:479–484. - PubMed
-
- Murray C. J. L., Vos T., Lozano R., Naghavi M., Flaxman A. D., Michaud C., Ezzati M., Shibuya K., Salomon J. A., Abdalla S., Aboyans V., Abraham J., Ackerman I., Aggarwal R., Ahn S. Y., Ali M. K., AlMazroa M. A., Alvarado M., Anderson H. R., Anderson L. M., Andrews K. G., Atkinson C., Baddour L. M., Bahalim A. N., Barker-Collo S., Barrero L. H., Bartels D. H., Basáñez M.-G., Baxter A., Bell M. L., Benjamin E. J., Bennett D., Bernabé E., Bhalla K., Bhandari B., Bikbov B., Bin Abdulhak A., Birbeck G., Black J. A., Blencowe H., Blore J. D., Blyth F., Bolliger I., Bonaventure A., Boufous S., Bourne R., Boussinesq M., Braithwaite T., Brayne C., Bridgett L., Brooker S., Brooks P., Brugha T. S., Bryan-Hancock C., Bucello C., Buchbinder R., Buckle G., Budke C. M., Burch M., Burney P., Burstein R., Calabria B., Campbell B., Canter C. E., Carabin H., Carapetis J., Carmona L., Cella C., Charlson F., Chen H., Cheng A. T.-A., Chou D., Chugh S. S., Coffeng L. E., Colan S. D., Colquhoun S., Colson K. E., Condon J., Connor M. D., Cooper L. T., Corriere M., Cortinovis M., de Vaccaro K. C., Couser W., Cowie B. C., Criqui M. H., Cross M., Dabhadkar K. C., Dahiya M., Dahodwala N., Damsere-Derry J., Danaei G., Davis A., De Leo D., Degenhardt L., Dellavalle R., Delossantos A., Denenberg J., Derrett S., Des Jarlais D. C., Dharmaratne S. D., Dherani M., Diaz-Torne C., Dolk H., Dorsey E. R., Driscoll T., Duber H., Ebel B., Edmond K., Elbaz A., Ali S. E., Erskine H., Erwin P. J., Espindola P., Ewoigbokhan S. E., Farzadfar F., Feigin V., Felson D. T., Ferrari A., Ferri C. P., Fèvre E. M., Finucane M. M., Flaxman S., Flood L., Foreman K., Forouzanfar M. H., Fowkes F. G. R., Fransen M., Freeman M. K., Gabbe B. J., Gabriel S. E., Gakidou E., Ganatra H. A., Garcia B., Gaspari F., Gillum R. F., Gmel G., Gonzalez-Medina D., Gosselin R., Grainger R., Grant B., Groeger J., Guillemin F., Gunnell D., Gupta R., Haagsma J., Hagan H., Halasa Y. A., Hall W., Haring D., Haro J. M., Harrison J. E., Havmoeller R., Hay R. J., Higashi H., Hill C., Hoen B., Hoffman H., Hotez P. J., Hoy D., Huang J. J., Ibeanusi S. E., Jacobsen K. H., James S. L., Jarvis D., Jasrasaria R., Jayaraman S., Johns N., Jonas J. B., Karthikeyan G., Kassebaum N., Kawakami N., Keren A., Khoo J.-P., King C. H., Knowlton L. M., Kobusingye O., Koranteng A., Krishnamurthi R., Laden F., Lalloo R., Laslett L. L., Lathlean T., Leasher J. L., Lee Y. Y., Leigh J., Levinson D., Lim S. S., Limb E., Lin J. K., Lipnick M., Lipshultz S. E., Liu W., Loane M., Ohno S. L., Lyons R., Mabweijano J., MacIntyre M. F., Malekzadeh R., Mallinger L., Manivannan S., Marcenes W., March L., Margolis D. J., Marks G. B., Marks R., Matsumori A., Matzopoulos R., Mayosi B. M., McAnulty J. H., McDermott M. M., McGill N., McGrath J., Medina-Mora M. E., Meltzer M., Memish Z. A., Mensah G. A., Merriman T. R., Meyer A.-C., Miglioli V., Miller M., Miller T. R., Mitchell P. B., Mock C., Mocumbi A. O., Moffitt T. E., Mokdad A. A., Monasta L., Montico M., Moradi-Lakeh M., Moran A., Morawska L., Mori R., Murdoch M. E., Mwaniki M. K., Naidoo K., Nair M. N., Naldi L., Narayan K. M. V., Nelson P. K., Nelson R. G., Nevitt M. C., Newton C. R., Nolte S., Norman P., Norman R., O'Donnell M., O'Hanlon S., Olives C., Omer S. B., Ortblad K., Osborne R., Ozgediz D., Page A., Pahari B., Pandian J. D., Rivero A. P., Patten S. B., Pearce N., Padilla R. P., Perez-Ruiz F., Perico N., Pesudovs K., Phillips D., Phillips M. R., Pierce K., Pion S., Polanczyk G. V., Polinder S., Pope C. A., Popova S., Porrini E., Pourmalek F., Prince M., Pullan R. L., Ramaiah K. D., Ranganathan D., Razavi H., Regan M., Rehm J. T., Rein D. B., Remuzzi G., Richardson K., Rivara F. P., Roberts T., Robinson C., De Leòn F. R., Ronfani L., Room R., Rosenfeld L. C., Rushton L., Sacco R. L., Saha S., Sampson U., Sanchez-Riera L., Sanman E., Schwebel D. C., Scott J. G., Segui-Gomez M., Shahraz S., Shepard D. S., Shin H., Shivakoti R., Silberberg D., Singh D., Singh G. M., Singh J. A., Singleton J., Sleet D. A., Sliwa K., Smith E., Smith J. L., Stapelberg N. J., Steer A., Steiner T., Stolk W. A., Stovner L. J., Sudfeld C., Syed S., Tamburlini G., Tavakkoli M., Taylor H. R., Taylor J. A., Taylor W. J., Thomas B., Thomson W. M., Thurston G. D., Tleyjeh I. M., Tonelli M., Towbin J. A., Truelsen T., Tsilimbaris M. K., Ubeda C., Undurraga E. A., van der Werf M. J., van Os J., Vavilala M. S., Venketasubramanian N., Wang M., Wang W., Watt K., Weatherall D. J., Weinstock M. A., Weintraub R., Weisskopf M. G., Weissman M. M., White R. A., Whiteford H., Wiebe N., Wiersma S. T., Wilkinson J. D., Williams H. C., Williams S. R., Witt E., Wolfe F., Woolf A. D., Wulf S., Yeh P.-H., Zaidi A. K., Zheng Z.-J., Zonies D., Lopez A. D. Lancet. 2012;380:2197–2223. - PubMed
-
- GBD Compare|IHME Viz Hub, https://vizhub.healthdata.org/gbd-compare/, (accessed 5 March 2019).
-
- Stroke Facts|cdc.gov, https://www.cdc.gov/stroke/facts.htm, (accessed 5 March 2019).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

