Scaffold hybridization strategy towards potent hydroxamate-based inhibitors of Flaviviridae viruses and Trypanosoma species
- PMID: 31303998
- PMCID: PMC6596085
- DOI: 10.1039/c9md00200f
Scaffold hybridization strategy towards potent hydroxamate-based inhibitors of Flaviviridae viruses and Trypanosoma species
Abstract
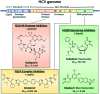
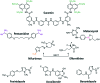
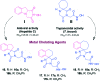
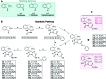
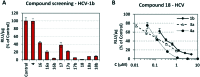
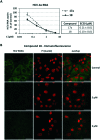
Infections with Flaviviridae viruses, such as hepatitis C virus (HCV) and dengue virus (DENV) pose global health threats. Infected individuals are at risk of developing chronic liver failure or haemorrhagic fever respectively, often with a fatal outcome if left untreated. Diseases caused by tropical parasites of the Trypanosoma species, T. brucei and T. cruzi, constitute significant socioeconomic burden in sub-Saharan Africa and continental Latin America, yet drug development is under-funded. Anti-HCV chemotherapy is associated with severe side effects and high cost, while dengue has no clinically approved therapy and antiparasitic drugs are outdated and difficult to administer. Moreover, drug resistance is an emerging concern. Consequently, the need for new revolutionary chemotherapies is urgent. By utilizing a molecular framework combination approach, we combined two distinct chemical entities with proven antiviral and trypanocidal activity into a novel hybrid scaffold attached by an acetohydroxamic acid group (CH2CONHOH), aiming at derivatives with dual activity. The novel spiro-carbocyclic substituted hydantoin analogues were rationally designed, synthesized and evaluated for their potency against three HCV genotypes (1b, 3a, 4a), DENV and two Trypanosoma species (T. brucei, T. cruzi). They exhibited significant EC50 values and remarkable selectivity indices. Several modifications were undertaken to further explore the structure activity relationships (SARs) and confirm the pivotal role of the acetohydroxamic acid metal binding group.
Figures
Similar articles
-
Novel Lipophilic Hydroxamates Based on Spirocarbocyclic Hydantoin Scaffolds with Potent Antiviral and Trypanocidal Activity.Pharmaceuticals (Basel). 2023 Jul 24;16(7):1046. doi: 10.3390/ph16071046. Pharmaceuticals (Basel). 2023. PMID: 37513957 Free PMC article.
-
Lipophilic Guanylhydrazone Analogues as Promising Trypanocidal Agents: An Extended SAR Study.Curr Pharm Des. 2020;26(8):838-866. doi: 10.2174/1381612826666200210150127. Curr Pharm Des. 2020. PMID: 32039675 Review.
-
Lipophilic conformationally constrained spiro carbocyclic 2,6-diketopiperazine-1-acetohydroxamic acid analogues as trypanocidal and leishmanicidal agents: An extended SAR study.Chem Biol Drug Des. 2018 Feb;91(2):408-421. doi: 10.1111/cbdd.13088. Epub 2017 Sep 18. Chem Biol Drug Des. 2018. PMID: 28834291
-
Synergy testing of FDA-approved drugs identifies potent drug combinations against Trypanosoma cruzi.PLoS Negl Trop Dis. 2014 Jul 17;8(7):e2977. doi: 10.1371/journal.pntd.0002977. eCollection 2014 Jul. PLoS Negl Trop Dis. 2014. PMID: 25033456 Free PMC article.
-
Diamidine activity against trypanosomes: the state of the art.Curr Mol Pharmacol. 2008 Jun;1(2):151-61. doi: 10.2174/1874467210801020151. Curr Mol Pharmacol. 2008. PMID: 20021429 Review.
Cited by
-
New Lipophilic Hydroxamates as Promising Trypanocidal Agents: Design, Synthesis, SAR, and Conformational Behavior Studies.ACS Med Chem Lett. 2024 Jun 6;15(7):1041-1048. doi: 10.1021/acsmedchemlett.4c00111. eCollection 2024 Jul 11. ACS Med Chem Lett. 2024. PMID: 39015276 Free PMC article.
-
Targeting Metalloenzymes: The "Achilles' Heel" of Viruses and Parasites.Pharmaceuticals (Basel). 2023 Jun 19;16(6):901. doi: 10.3390/ph16060901. Pharmaceuticals (Basel). 2023. PMID: 37375848 Free PMC article. Review.
-
Novel Pyrazino[1,2-a]indole-1,3(2H,4H)-dione Derivatives Targeting the Replication of Flaviviridae Viruses: Structural and Mechanistic Insights.Viruses. 2024 Aug 1;16(8):1238. doi: 10.3390/v16081238. Viruses. 2024. PMID: 39205212 Free PMC article.
-
Antiviral Activity of an Indole-Type Compound Derived from Natural Products, Identified by Virtual Screening by Interaction on Dengue Virus NS5 Protein.Viruses. 2023 Jul 17;15(7):1563. doi: 10.3390/v15071563. Viruses. 2023. PMID: 37515249 Free PMC article.
-
Novel 6-Aminoquinazolinone Derivatives as Potential Cross GT1-4 HCV NS5B Inhibitors.Viruses. 2022 Dec 12;14(12):2767. doi: 10.3390/v14122767. Viruses. 2022. PMID: 36560772 Free PMC article.
References
-
- World Health Organization, Hepatitis C, http://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed March 2019).
-
- Bartenschlager R., Lohmann V., Penin F. Nat. Rev. Microbiol. 2013;11:482–496. - PubMed
-
- Moradpour D., Penin F., Rice C. M. Nat. Rev. Microbiol. 2007;5:453–463. - PubMed
-
- Ramirez S., Bukh J. Antiviral Res. 2018;158:264–287. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

