LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis
- PMID: 31304004
- PMCID: PMC6600894
- DOI: 10.1186/s13578-019-0302-2
LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis
Abstract
Background: Many studies have reported that long noncoding RNAs (lncRNAs) could act as sponges for microRNAs (miRNAs) and play important roles in the regulation of osteoarthritis (OA). Yet, the underlying mechanisms of lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) in OA are still unclear. Therefore, we aimed to explore the regulation mechanisms of MALAT1 in OA procession.
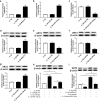
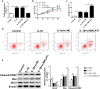
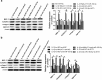
Methods: IL-1β treatment in chondrocyte was used to mimic OA in vitro. MALAT1, miR-150-5p and AKT3 expression levels were detected via qRT-PCR. The protein levels of AKT3, MMP-13, ADAMTS-5, Bax, Bcl-2, cleaved-PARP, collagen II and aggracan were measured by western blot. MTT assay was performed to detect cell proliferation ability. The apoptosis of chondrocytes was determined using flow cytometry and western blot. Luciferase assay and RNA immunoprecipitation (RIP) assays were used to confirm the relationship among MALAT1, miR-150-5p and AKT3.
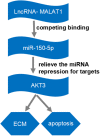
Results: In our study, MALAT1 and AKT3 were upregulated while miR-150-5p was downregulated in OA in vitro and vivo. The level of miR-150-5p was negatively correlated with that of MALAT1 or AKT3. More importantly, overexpression of MALAT1 promoted the expression of AKT3 by negatively regulating miR-150-5p. MALAT1 knockdown inhibited cell proliferation, promoted apoptosis, increased MMP-13, ADAMTS-5 expression and decreased collagen II, aggracan expression in IL-1β treated chondrocytes. MALAT1 upregulation or AKT3 overexpression enhanced proliferation, inhibited apoptosis and extracellular matrix (ECM) degradation, which was undermined by overexpression of miR-150-5p. By contrast, miR-150-5p depletion rescued the effect of MALAT1 downregulation or loss of AKT3 on IL-1β-stimulated chondrocytes.
Conclusion: MALAT1 was responsible for cell proliferation, apoptosis, and ECM degradation via miR-150-5p/AKT3 axis.
Keywords: AKT3; MALAT1; MALAT1/miR-150-5p/AKT3; OA; miR-150-5p.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures




Similar articles
-
LncRNA MALAT1/MiR-145 Adjusts IL-1β-Induced Chondrocytes Viability and Cartilage Matrix Degradation by Regulating ADAMTS5 in Human Osteoarthritis.Yonsei Med J. 2019 Nov;60(11):1081-1092. doi: 10.3349/ymj.2019.60.11.1081. Yonsei Med J. 2019. PMID: 31637891 Free PMC article.
-
LncRNA SNHG5 promotes chondrocyte proliferation and inhibits apoptosis in osteoarthritis by regulating miR-10a-5p/H3F3B axis.Connect Tissue Res. 2021 Nov;62(6):605-614. doi: 10.1080/03008207.2020.1825701. Epub 2020 Sep 23. Connect Tissue Res. 2021. PMID: 32967481
-
LncRNA PVT1 regulates biological function of osteoarthritis cells by regulating miR-497/AKT3 axis.Medicine (Baltimore). 2022 Nov 11;101(45):e31725. doi: 10.1097/MD.0000000000031725. Medicine (Baltimore). 2022. PMID: 36397317 Free PMC article.
-
CircRNA circ-IQGAP1 Knockdown Alleviates Interleukin-1β-Induced Osteoarthritis Progression via Targeting miR-671-5p/TCF4.Orthop Surg. 2021 May;13(3):1036-1046. doi: 10.1111/os.12923. Epub 2021 Mar 5. Orthop Surg. 2021. PMID: 33675175 Free PMC article.
-
LncRNA ZFAS1 protects chondrocytes from IL-1β-induced apoptosis and extracellular matrix degradation via regulating miR-7-5p/FLRT2 axis.J Orthop Surg Res. 2023 Apr 25;18(1):320. doi: 10.1186/s13018-023-03802-9. J Orthop Surg Res. 2023. PMID: 37098630 Free PMC article.
Cited by
-
CircSCAPER contributes to IL-1β-induced osteoarthritis in vitro via miR-140-3p/EZH2 axis.Bone Joint Res. 2022 Feb;11(2):61-72. doi: 10.1302/2046-3758.112.BJR-2020-0482.R2. Bone Joint Res. 2022. PMID: 35103493 Free PMC article.
-
miR-150-5p inhibits osteogenic differentiation of fibroblasts in ankylosing spondylitis by targeting VDR.Exp Ther Med. 2022 Apr;23(4):283. doi: 10.3892/etm.2022.11213. Epub 2022 Feb 15. Exp Ther Med. 2022. PMID: 35317439 Free PMC article.
-
The Role of Mitochondrial Metabolism, AMPK-SIRT Mediated Pathway, LncRNA and MicroRNA in Osteoarthritis.Biomedicines. 2022 Jun 22;10(7):1477. doi: 10.3390/biomedicines10071477. Biomedicines. 2022. PMID: 35884782 Free PMC article. Review.
-
LncRNA MINCR attenuates osteoarthritis progression via sponging miR-146a-5p to promote BMPR2 expression.Cell Cycle. 2022 Nov;21(22):2417-2432. doi: 10.1080/15384101.2022.2099191. Epub 2022 Jul 18. Cell Cycle. 2022. PMID: 35848848 Free PMC article.
-
Crosstalk Among circRNA/lncRNA, miRNA, and mRNA in Osteoarthritis.Front Cell Dev Biol. 2021 Dec 15;9:774370. doi: 10.3389/fcell.2021.774370. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34977024 Free PMC article. Review.
References
-
- Berenbaum F. Osteoarthritis is an inflammatory disease. Osteoarthritis Cartilage. 2012;20:S5. - PubMed
-
- Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Prac Res Clin Rheumatol. 2006;20:3–25. - PubMed
-
- Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis—an untreatable disease? Nat Rev Drug Discov. 2005;4:331–344. - PubMed
-
- Okada A, Okada Y. Progress of research in osteoarthritis. Metalloproteinases in osteoarthritis. Clin Calcium. 2009;19:1593–1601. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

