Recycling of End-of-Life Poly(bisphenol A carbonate) via Alkali Metal Halide-Catalyzed Phenolysis
- PMID: 31304075
- PMCID: PMC6604237
- DOI: 10.1002/open.201900149
Recycling of End-of-Life Poly(bisphenol A carbonate) via Alkali Metal Halide-Catalyzed Phenolysis
Abstract
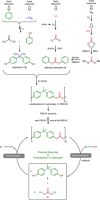
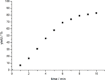
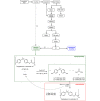
The chemical recycling of end-of-life plastic waste streams can contribute to a resource-conserving and sustainable society. This matter of recycling is composed of a sequence of depolymerization and subsequent polymerization reactions. In this regard, we have studied the chemical recycling of end-of-life poly(bisphenol A carbonate) applying phenol as depolymerization reagent. In the presence of catalytic amounts of alkali metal halides as products bisphenol A and diphenyl carbonate were obtained in excellent turnover frequencies of up to 1392 h-1 and short reaction times. These depolymerization products offer the straightforward possibility to close the cycle by producing new poly(bisphenol A carbonate) and as second product phenol, which can be reused for further depolymerizations.
Keywords: catalysis; depolymerization; green chemistry; polymers; recycling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Plastics are typically composed of polymers and often other substances e. g. fillers, plasticizers, colorants.
-
- Landfill storage depends strongly on local regulations, e. g. in the European Union landfill storage has been limited to the necessary minimum (Landfill directive; see for instance:
-
- Burnley S., Resour. Conserv. Recycl. 2001, 32, 349–358;
-
- http://ec.europa.eu/environment/waste/landfill_index.htm (08. 03. 2019);
LinkOut - more resources
Full Text Sources

