Demographics and survival of patients with idiopathic pulmonary fibrosis in the FinnishIPF registry
- PMID: 31304177
- PMCID: PMC6612605
- DOI: 10.1183/23120541.00170-2018
Demographics and survival of patients with idiopathic pulmonary fibrosis in the FinnishIPF registry
Abstract
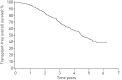
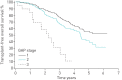
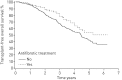
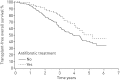
Idiopathic pulmonary fibrosis (IPF) is characterised by unpredictable disease course and poor survival. After the introduction of novel antifibrotic drugs, the prognosis of patients with IPF is probably changing. FinnishIPF, a nationwide registry of carefully characterised patients, was initiated in Finland in 2011. For the data analysis, we included 453 incident IPF patients diagnosed during 2011-2015. In this study, we describe the demographics and prognosis of these real-life patients. The median overall survival time of registered IPF patients was 4.5 years. The transplant-free survival at 1, 2, 3, 4 and 5 years was 95%, 83%, 70%, 58% and 45%, respectively. Smoking did not have any effect on survival. 117 (26%) patients received pirfenidone or nintedanib. Patients who received ≥6 months of treatment had better survival compared with those who did not receive treatment but this difference disappeared after age adjustment. The transplantation rate was 3%. Although IPF is diagnosed in Finland at a older age, the prognosis is better than expected due to a relatively well preserved lung function at diagnosis. Age and pulmonary function were identified as independent predictors of survival in the entire IPF patient population as well as in patients who had received antifibrotic treatment.
Conflict of interest statement
Conflict of interest: J. Kaunisto reports lecture fees from Boehringer and Roche, and support to attend an international conference from Sanofi Genzyme, outside the submitted work. Conflict of interest: E-R. Salomaa has nothing to disclose. Conflict of interest: U. Hodgson has nothing to disclose. Conflict of interest: R. Kaarteenaho reports grants from the Foundation of the Finnish Anti-Tuberculosis Association, the Research Foundation of the Pulmonary Diseases, Jalmari and Rauha Ahokas Foundation and the Research Foundation of North Finland, lecture fees and an advisory board fee from GlaxoSmithKline, Roche and Boehringer-Ingelheim, and conference travel costs from Orion Pharma, outside the submitted work. Conflict of interest: H. Kankaanranta reports fees for lectures and consulting, costs for attending an international conference, and a research grant from AstraZeneca; fees for consulting from Chiesi Pharma AB; fees for lectures and consulting, and costs for attending and international conference from Boehringer-Ingelheim; fees for lectures and consulting from Novartis; fees for lectures from Mundipharma; fees for consulting from GlaxoSmithKline; fees for lectures and consulting from Orion Pharma; and fees for consulting from SanofiGenzyme, all outside the submitted work. Conflict of interest: K. Koli has nothing to disclose. Conflict of interest: T. Vahlberg has nothing to disclose. Conflict of interest: M. Myllärniemi has nothing to disclose.
Figures
References
-
- Raghu G, Chen SY, Yeh WS, et al. . Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med 2014; 2: 566–567. - PubMed
-
- King TE Jr, Albera C, Bradford WZ, et al. . All-cause mortality rate in patients with idiopathic pulmonary fibrosis. Implications for the design and execution of clinical trials. Am J Respir Crit Care Med 2014; 189: 825–831. - PubMed
-
- Marshall DC, Salciccioli JD, Shea BS, et al. . Trends in mortality from idiopathic pulmonary fibrosis in the European Union: an observational study of the WHO mortality database from 2001–2013. Eur Respir J 2018; 51: 1701603. - PubMed
-
- Kim HJ, Perlman D, Tomic R. Natural history of idiopathic pulmonary fibrosis. Respir Med 2015; 109: 661–670. - PubMed
LinkOut - more resources
Full Text Sources
