Sex differences in neural stress responses and correlation with subjective stress and stress regulation
- PMID: 31304198
- PMCID: PMC6603439
- DOI: 10.1016/j.ynstr.2019.100177
Sex differences in neural stress responses and correlation with subjective stress and stress regulation
Abstract
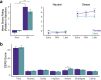
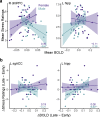
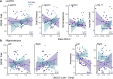
Emotional stress responses, encompassing both stress reactivity and regulation, have been shown to differ between men and women, but the neural networks supporting these processes remain unclear. The current study used functional neuroimaging (fMRI) to investigate sex differences in neural responses during stress and the sex-specific relationships between these responses and emotional stress responses for men and women. A significant sex by condition interaction revealed that men showed greater stress responses in prefrontal cortex (PFC) regions, whereas women had stronger responses in limbic/striatal regions. Although men and women did not significantly differ in emotional stress reactivity or subjective reports of stress regulation, these responses were associated with distinct neural networks. Higher dorsomedial PFC responses were associated with lower stress reactivity in men, but higher stress reactivity in women. In contrast, while higher ventromedial PFC stress responses were associated with worse stress regulation in men (but better regulation in women), dynamic increases in vmPFC responses during stress were associated with lower stress reactivity in men. Finally, stress-induced hippocampal responses were more adaptive for women: for men, high and dynamically increasing responses in left hippocampus were associated with high stress reactivity, and dynamic increases in the left (but not right) hippocampus were associated with worse stress regulation. Together, these results reveal that men and women engage distinct neural networks during stress, and sex-specific neural stress responses facilitate optimal emotional stress responses.
Keywords: Emotion; Hippocampus; Medial prefrontal cortex; Sex; Stress; fMRI.
Figures

Similar articles
-
Dynamic neural activity during stress signals resilient coping.Proc Natl Acad Sci U S A. 2016 Aug 2;113(31):8837-42. doi: 10.1073/pnas.1600965113. Epub 2016 Jul 18. Proc Natl Acad Sci U S A. 2016. PMID: 27432990 Free PMC article.
-
Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies.Neuropsychologia. 2012 Jun;50(7):1578-93. doi: 10.1016/j.neuropsychologia.2012.03.011. Epub 2012 Mar 17. Neuropsychologia. 2012. PMID: 22450197
-
Anger Modulates Influence Hierarchies Within and Between Emotional Reactivity and Regulation Networks.Front Behav Neurosci. 2018 Apr 4;12:60. doi: 10.3389/fnbeh.2018.00060. eCollection 2018. Front Behav Neurosci. 2018. PMID: 29681803 Free PMC article.
-
Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine.Stress. 2004 Jun;7(2):131-43. doi: 10.1080/102538900410001679310. Stress. 2004. PMID: 15512858 Review.
-
Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner.Psychoneuroendocrinology. 2010 Jan;35(1):179-91. doi: 10.1016/j.psyneuen.2009.02.016. Psychoneuroendocrinology. 2010. PMID: 19362426 Review.
Cited by
-
Influence of social support and coping strategies on psychological stress among frontline medical personnel during the Yangbi Earthquake: a cross-sectional analysis.Front Psychiatry. 2024 Sep 27;15:1473005. doi: 10.3389/fpsyt.2024.1473005. eCollection 2024. Front Psychiatry. 2024. PMID: 39398955 Free PMC article.
-
Psychometric properties of the Arabic Stress Numerical Rating Scale (SNRS-11) in adolescents.Sci Rep. 2024 Dec 2;14(1):29862. doi: 10.1038/s41598-024-81554-0. Sci Rep. 2024. PMID: 39622924 Free PMC article.
-
Sex differences in discrimination behavior and orbitofrontal engagement during context-gated reward prediction.Elife. 2024 Jul 24;12:RP93509. doi: 10.7554/eLife.93509. Elife. 2024. PMID: 39046898 Free PMC article.
-
Stress responses in high-fidelity simulation and standard simulation training among medical students.BMC Med Educ. 2023 Feb 17;23(1):116. doi: 10.1186/s12909-023-04101-x. BMC Med Educ. 2023. PMID: 36797725 Free PMC article.
-
Sex-specific neural responses to acute psychosocial stress in depression.Transl Psychiatry. 2022 Jan 10;12(1):2. doi: 10.1038/s41398-021-01768-y. Transl Psychiatry. 2022. PMID: 35013110 Free PMC article.
References
-
- Babor T.F., de la Fuente J.R., Saunders J., Grant M. World Health Organization; Geneva, Switzerland: 1992. The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Health Care.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

