Chronic unpredictable stress influenced the behavioral but not the neurodegenerative impact of paraquat
- PMID: 31304199
- PMCID: PMC6599913
- DOI: 10.1016/j.ynstr.2019.100179
Chronic unpredictable stress influenced the behavioral but not the neurodegenerative impact of paraquat
Abstract
The impact of psychological stressors on the progression of motor and non-motor disturbances observed in Parkinson's disease (PD) has received little attention. Given that PD likely results from many different environmental "hits", we were interested in whether a chronic unpredictable stressor regimen would act additively or possibly even synergistically to augment the impact of the toxicant, paraquat, which has previously been linked to PD. Our findings support the contention that paraquat itself acted as a systemic stressor, with the pesticide increasing plasma corticosterone, as well as altering glucocorticoid receptor (GR) expression in the hippocampus. Furthermore, stressed mice that also received paraquat displayed synergistic motor coordination impairment on a rotarod test and augmented signs of anhedonia (sucrose preference test). The individual stressor and paraquat treatments also caused a range of non-motor (e.g. open field, Y and plus mazes) deficits, but there were no signs of an interaction (neither additive nor synergistic) between the insults. Similarly, paraquat caused the expected loss of substantia nigra dopamine neurons and microglial activation, but this effect was not further influenced by the chronic stressor. Taken together, these results indicate that paraquat has many effects comparable to that of a more traditional stressor and that at least some behavioral measures (i.e. sucrose preference and rotarod) are augmented by the combined pesticide and stress treatments. Thus, although psychological stressors might not necessarily increase the neurodegenerative effects of the toxicant exposure, they may promote co-morbid behaviors pathology.
Keywords: AAR, alternate arm return; ANOVA, analysis of variance; BCA, bicinchoninic acid; BDNF, brain derived neurotrophic factor; CUS, chronic unpredictable stress; Cytokine; EDTA, ethylenediaminetetraacetic acid; ELISA, enzyme-linked immunosorbent assay; EPM, elevated plus maze; FST, forced swim test; GR, glucocorticoid receptor; HPA, hypothalamus-pituitary adrenal; IBA1, ionized calcium-binding adapter molecule 1; Inflammatory; MMx, Micromax; Microglia; PB, phosphate buffer; PBS, phosphate buffered saline; PD, Parkinson's disease; PFA, paraformaldehyde; PVDF, polyvinylidene difluoride; Parkinson's; RIPA, Radio Immuno Precipitation Assay; RR, rotarod; SAB, spontaneous alternation behavior; SAR, same arm return; SDS, sodium dodecyl sulphate; SNc, substantia nigra pars compacta; SPT, sucrose preference test; Stress; TH, tyrosine hydroxylase; Toxicity; VTA, ventral tegmental area; pGR, phosphate glucocorticoid receptor.
Figures
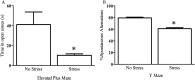
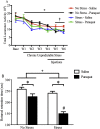


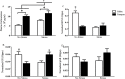
Similar articles
-
Paraquat and psychological stressor interactions as pertains to Parkinsonian co-morbidity.Neurobiol Stress. 2015 Nov 12;2:85-93. doi: 10.1016/j.ynstr.2015.09.001. eCollection 2015. Neurobiol Stress. 2015. PMID: 26844243 Free PMC article.
-
Granulocyte macrophage-colony stimulating factor protects against substantia nigra dopaminergic cell loss in an environmental toxin model of Parkinson's disease.Neurobiol Dis. 2011 Jul;43(1):99-112. doi: 10.1016/j.nbd.2011.02.011. Epub 2011 Mar 4. Neurobiol Dis. 2011. PMID: 21377529
-
Characterizing the protracted neurobiological and neuroanatomical effects of paraquat in a murine model of Parkinson's disease.Neurobiol Aging. 2021 Apr;100:11-21. doi: 10.1016/j.neurobiolaging.2020.11.013. Epub 2020 Nov 26. Neurobiol Aging. 2021. PMID: 33450723
-
Age and Chronicity of Administration Dramatically Influenced the Impact of Low Dose Paraquat Exposure on Behavior and Hypothalamic-Pituitary-Adrenal Activity.Front Aging Neurosci. 2017 Jul 14;9:222. doi: 10.3389/fnagi.2017.00222. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28769783 Free PMC article.
-
Environment- and activity-dependent dopamine neurotransmitter plasticity in the adult substantia nigra.J Chem Neuroanat. 2016 Apr;73:21-32. doi: 10.1016/j.jchemneu.2015.12.009. Epub 2015 Dec 21. J Chem Neuroanat. 2016. PMID: 26718607 Review.
Cited by
-
Effects of the Pharmabiotic U-21 under Conditions of a Combined Neuroinflammatory Model of Parkinson's Disease in Rats.Bull Exp Biol Med. 2024 Jun;177(2):225-230. doi: 10.1007/s10517-024-06161-5. Epub 2024 Aug 2. Bull Exp Biol Med. 2024. PMID: 39093470
-
Loss of p21-activated kinase Mbt/PAK4 causes Parkinson-like phenotypes in Drosophila.Dis Model Mech. 2021 Jun 1;14(6):dmm047811. doi: 10.1242/dmm.047811. Epub 2021 Jun 14. Dis Model Mech. 2021. PMID: 34125184 Free PMC article.
-
The role of allopregnanolone in depressive-like behaviors: Focus on neurotrophic proteins.Neurobiol Stress. 2020 Apr 9;12:100218. doi: 10.1016/j.ynstr.2020.100218. eCollection 2020 May. Neurobiol Stress. 2020. PMID: 32435667 Free PMC article.
-
Behavioral Tests in Neurotoxin-Induced Animal Models of Parkinson's Disease.Antioxidants (Basel). 2020 Oct 16;9(10):1007. doi: 10.3390/antiox9101007. Antioxidants (Basel). 2020. PMID: 33081318 Free PMC article. Review.
-
Effect of cyanocobalamin (vitamin B12) on paraquat-induced brain injury in mice.Iran J Basic Med Sci. 2022 Jun;25(6):745-754. doi: 10.22038/IJBMS.2022.64164.14128. Iran J Basic Med Sci. 2022. PMID: 35949307 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

