Determination of triacylglycerol oxidation mechanisms in canola oil using liquid chromatography-tandem mass spectrometry
- PMID: 31304251
- PMCID: PMC6550225
- DOI: 10.1038/s41538-017-0009-x
Determination of triacylglycerol oxidation mechanisms in canola oil using liquid chromatography-tandem mass spectrometry
Abstract
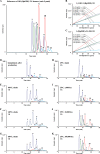
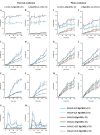
Triacylglycerol (TG), the main component of edible oil, is oxidized by thermal- or photo- oxidation to form TG hydroperoxide (TGOOH) as the primary oxidation product. Since TGOOH and its subsequent oxidation products cause not only the deterioration of oil quality but also various toxicities, preventing the oxidation of edible oils is essential. Therefore understanding oxidation mechanisms that cause the formation of TGOOH is necessary. Since isomeric information of lipid hydroperoxide provides insights about oil oxidation mechanisms, we focused on dioleoyl-(hydroperoxy octadecadienoyl)-TG (OO-HpODE-TG) isomers, which are the primary oxidation products of the most abundant TG molecular species (dioleoyl-linoleoyl-TG) in canola oil. To secure highly selective and sensitive analysis, authentic OO-HpODE-TG isomer references (i.e., hydroperoxide positional/geometrical isomers) were synthesized and analyzed with HPLC-MS/MS. With the use of the method, photo- or thermal- oxidized edible oils were analyzed. While dioleoyl-(10-hydroperoxy-8E,12Z-octadecadienoyl)-TG (OO-(10-HpODE)-TG) and dioleoyl-(12-hydroperoxy-9Z,13E-octadecadienoyl)-TG (OO-(12-HpODE)-TG) were characteristically detected in photo-oxidized oils, dioleoyl-(9-hydroperoxy-10E,12E-octadecadienoyl)-TG and dioleoyl-(13-hydroperoxy-9E,11E-octadecadienoyl)-TG were found to increase depending on temperature in thermal-oxidized oils. These results prove that our methods not only evaluate oil oxidation in levels that are unquantifiable with peroxide value, but also allows for the determination of oil oxidation mechanisms. From the analysis of marketed canola oils, photo-oxidized products (i.e., OO-(10-HpODE)-TG and OO-(12-HpODE)-TG) were characteristically accumulated compared to the oil analyzed immediately after production. The method described in this paper is valuable in the understanding of oil and food oxidation mechanisms, and may be applied to the development of preventive methods against food deterioration.
Keywords: Industry; Technology.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing financial interests.
Figures




Similar articles
-
A novel chiral stationary phase LC-MS/MS method to evaluate oxidation mechanisms of edible oils.Sci Rep. 2017 Aug 30;7(1):10026. doi: 10.1038/s41598-017-10536-2. Sci Rep. 2017. PMID: 28855636 Free PMC article.
-
Tandem mass spectrometry analysis of linoleic and arachidonic acid hydroperoxides via promotion of alkali metal adduct formation.Anal Chem. 2015;87(9):4980-7. doi: 10.1021/acs.analchem.5b00851. Epub 2015 Apr 24. Anal Chem. 2015. PMID: 25874840
-
Elucidation of Olive Oil Oxidation Mechanisms by Analysis of Triacylglycerol Hydroperoxide Isomers Using LC-MS/MS.Molecules. 2022 Aug 18;27(16):5282. doi: 10.3390/molecules27165282. Molecules. 2022. PMID: 36014520 Free PMC article.
-
A novel chiral stationary phase HPLC-MS/MS method to discriminate between enzymatic oxidation and auto-oxidation of phosphatidylcholine.Anal Bioanal Chem. 2016 Nov;408(27):7785-7793. doi: 10.1007/s00216-016-9882-4. Epub 2016 Aug 22. Anal Bioanal Chem. 2016. PMID: 27549797
-
Mechanism, indexes, methods, challenges, and perspectives of edible oil oxidation analysis.Crit Rev Food Sci Nutr. 2023;63(21):4901-4915. doi: 10.1080/10408398.2021.2009437. Epub 2021 Nov 30. Crit Rev Food Sci Nutr. 2023. PMID: 34845958 Review.
Cited by
-
Chemical Compositional Changes in Over-Oxidized Fish Oils.Foods. 2020 Oct 20;9(10):1501. doi: 10.3390/foods9101501. Foods. 2020. PMID: 33092165 Free PMC article.
-
Analytical and Structural Tools of Lipid Hydroperoxides: Present State and Future Perspectives.Molecules. 2022 Mar 25;27(7):2139. doi: 10.3390/molecules27072139. Molecules. 2022. PMID: 35408537 Free PMC article. Review.
-
Lipid hydroperoxides in nutrition, health, and diseases.Proc Jpn Acad Ser B Phys Biol Sci. 2021;97(4):161-196. doi: 10.2183/pjab.97.010. Proc Jpn Acad Ser B Phys Biol Sci. 2021. PMID: 33840675 Free PMC article. Review.
-
Determination of acrolein generation pathways from linoleic acid and linolenic acid: increment by photo irradiation.NPJ Sci Food. 2022 Apr 12;6(1):21. doi: 10.1038/s41538-022-00138-2. NPJ Sci Food. 2022. PMID: 35413955 Free PMC article.
-
Imaging and Analysis of Isomeric Unsaturated Lipids through Online Photochemical Derivatization of Carbon-Carbon Double Bonds*.Angew Chem Int Ed Engl. 2021 Mar 29;60(14):7559-7563. doi: 10.1002/anie.202016734. Epub 2021 Feb 26. Angew Chem Int Ed Engl. 2021. PMID: 33460514 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

