Suppression of olfactory signal transduction by insecticides
- PMID: 31304281
- PMCID: PMC6550234
- DOI: 10.1038/s41538-019-0042-z
Suppression of olfactory signal transduction by insecticides
Abstract
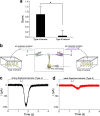
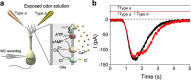
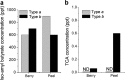
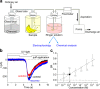
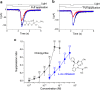
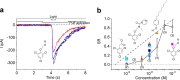
2,4,6-Trichloroanisole (TCA) is a well-known, potent off-flavour compound present in various foods and beverages. TCA has been hypothesised to be a universal cause of flavour loss experienced in daily life. Here, however, we show that titres for the suppression of olfactory transducer channels caused by low-quality bananas are much higher than those for that caused by the TCA itself contained in the banana. We resurveyed other components of low-quality bananas and found that bananas also contain an insecticide (chlorpyrifos), and that it suppresses olfactory transducer channels. Other insecticides also suppressed olfactory transducer channels. Hence, even after passing safety examinations, certain insecticides may decrease the quality of foods and beverages by reducing their intrinsic scents.
Keywords: Cyclic nucleotide-gated cation channels; Inhibition; Olfactory receptors.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures


Similar articles
-
2,4,6-trichloroanisole is a potent suppressor of olfactory signal transduction.Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):16235-40. doi: 10.1073/pnas.1300764110. Epub 2013 Sep 16. Proc Natl Acad Sci U S A. 2013. PMID: 24043819 Free PMC article.
-
Odorant inhibition of the olfactory cyclic nucleotide-gated channel with a native molecular assembly.J Gen Physiol. 2006 Sep;128(3):365-71. doi: 10.1085/jgp.200609577. J Gen Physiol. 2006. PMID: 16940558 Free PMC article.
-
Cyclic-nucleotide-gated cation current and Ca2+-activated Cl current elicited by odorant in vertebrate olfactory receptor neurons.Proc Natl Acad Sci U S A. 2016 Oct 4;113(40):11078-11087. doi: 10.1073/pnas.1613891113. Epub 2016 Sep 19. Proc Natl Acad Sci U S A. 2016. PMID: 27647918 Free PMC article.
-
Cyclic nucleotide-gated channels in visual and olfactory transduction.Biophys Chem. 1995 Aug;55(3):185-96. doi: 10.1016/0301-4622(94)00153-b. Biophys Chem. 1995. PMID: 7542935 Review.
-
Regulation of cyclic nucleotide-gated channels.Curr Opin Neurobiol. 2005 Jun;15(3):343-9. doi: 10.1016/j.conb.2005.05.014. Curr Opin Neurobiol. 2005. PMID: 15922582 Review.
Cited by
-
Olfactory cilia, regulation and control of olfaction.Physiol Rep. 2024 Oct;12(19):e70057. doi: 10.14814/phy2.70057. Physiol Rep. 2024. PMID: 39358841 Free PMC article. Review.
-
Segregation of Ca2+ signaling in olfactory signal transduction.J Gen Physiol. 2023 Apr 3;155(4):e202213165. doi: 10.1085/jgp.202213165. Epub 2023 Feb 14. J Gen Physiol. 2023. PMID: 36787110 Free PMC article.
References
-
- Buser HR, Zanier C, Tanner H. Identification of 2,4,6-trichloroanisole as a potent compound causing cork taint in wine. J. Agric. Food Chem. 1982;30:359–362. doi: 10.1021/jf00110a037. - DOI
LinkOut - more resources
Full Text Sources

