Wearable sensors for Parkinson's disease: which data are worth collecting for training symptom detection models
- PMID: 31304341
- PMCID: PMC6550186
- DOI: 10.1038/s41746-018-0071-z
Wearable sensors for Parkinson's disease: which data are worth collecting for training symptom detection models
Abstract
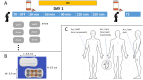
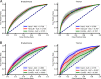
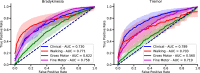
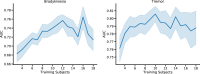
Machine learning algorithms that use data streams captured from soft wearable sensors have the potential to automatically detect PD symptoms and inform clinicians about the progression of disease. However, these algorithms must be trained with annotated data from clinical experts who can recognize symptoms, and collecting such data are costly. Understanding how many sensors and how much labeled data are required is key to successfully deploying these models outside of the clinic. Here we recorded movement data using 6 flexible wearable sensors in 20 individuals with PD over the course of multiple clinical assessments conducted on 1 day and repeated 2 weeks later. Participants performed 13 common tasks, such as walking or typing, and a clinician rated the severity of symptoms (bradykinesia and tremor). We then trained convolutional neural networks and statistical ensembles to detect whether a segment of movement showed signs of bradykinesia or tremor based on data from tasks performed by other individuals. Our results show that a single wearable sensor on the back of the hand is sufficient for detecting bradykinesia and tremor in the upper extremities, whereas using sensors on both sides does not improve performance. Increasing the amount of training data by adding other individuals can lead to improved performance, but repeating assessments with the same individuals-even at different medication states-does not substantially improve detection across days. Our results suggest that PD symptoms can be detected during a variety of activities and are best modeled by a dataset incorporating many individuals.
Keywords: Diagnostic markers; Parkinson's disease; Rehabilitation.
Conflict of interest statement
Competing interestsJ.A.R. and R.G. both hold equity in the company MC10 that makes wearable devices for medical applications. The remaining authors declare no competing interests.
Figures
Similar articles
-
Role of data measurement characteristics in the accurate detection of Parkinson's disease symptoms using wearable sensors.J Neuroeng Rehabil. 2020 Apr 20;17(1):52. doi: 10.1186/s12984-020-00684-4. J Neuroeng Rehabil. 2020. PMID: 32312287 Free PMC article.
-
Quantification of whole-body bradykinesia in Parkinson's disease participants using multiple inertial sensors.J Neurol Sci. 2018 Apr 15;387:157-165. doi: 10.1016/j.jns.2018.02.001. Epub 2018 Feb 2. J Neurol Sci. 2018. PMID: 29571855
-
Continuous and Unconstrained Tremor Monitoring in Parkinson's Disease Using Supervised Machine Learning and Wearable Sensors.Parkinsons Dis. 2024 May 20;2024:5787563. doi: 10.1155/2024/5787563. eCollection 2024. Parkinsons Dis. 2024. PMID: 38803413 Free PMC article.
-
Monitoring Motor Symptoms During Activities of Daily Living in Individuals With Parkinson's Disease.Front Neurol. 2018 Dec 12;9:1036. doi: 10.3389/fneur.2018.01036. eCollection 2018. Front Neurol. 2018. PMID: 30619024 Free PMC article. Review.
-
Assessing Gait in Parkinson's Disease Using Wearable Motion Sensors: A Systematic Review.Diseases. 2019 Feb 5;7(1):18. doi: 10.3390/diseases7010018. Diseases. 2019. PMID: 30764502 Free PMC article. Review.
Cited by
-
Detecting the symptoms of Parkinson's disease with non-standard video.J Neuroeng Rehabil. 2024 May 3;21(1):72. doi: 10.1186/s12984-024-01362-5. J Neuroeng Rehabil. 2024. PMID: 38702705 Free PMC article.
-
Benchmark on a large cohort for sleep-wake classification with machine learning techniques.NPJ Digit Med. 2019 Jun 7;2:50. doi: 10.1038/s41746-019-0126-9. eCollection 2019. NPJ Digit Med. 2019. PMID: 31304396 Free PMC article.
-
Parkinson Disease Recognition Using a Gamified Website: Machine Learning Development and Usability Study.JMIR Form Res. 2023 Sep 29;7:e49898. doi: 10.2196/49898. JMIR Form Res. 2023. PMID: 37773607 Free PMC article.
-
Role of data measurement characteristics in the accurate detection of Parkinson's disease symptoms using wearable sensors.J Neuroeng Rehabil. 2020 Apr 20;17(1):52. doi: 10.1186/s12984-020-00684-4. J Neuroeng Rehabil. 2020. PMID: 32312287 Free PMC article.
-
Skin sensors are the future of health care.Nature. 2019 Jul;571(7765):319-321. doi: 10.1038/d41586-019-02143-0. Nature. 2019. PMID: 31316200 No abstract available.
References
Grants and funding
LinkOut - more resources
Full Text Sources

