Deep learning enables robust assessment and selection of human blastocysts after in vitro fertilization
- PMID: 31304368
- PMCID: PMC6550169
- DOI: 10.1038/s41746-019-0096-y
Deep learning enables robust assessment and selection of human blastocysts after in vitro fertilization
Abstract
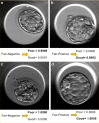
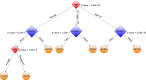
Visual morphology assessment is routinely used for evaluating of embryo quality and selecting human blastocysts for transfer after in vitro fertilization (IVF). However, the assessment produces different results between embryologists and as a result, the success rate of IVF remains low. To overcome uncertainties in embryo quality, multiple embryos are often implanted resulting in undesired multiple pregnancies and complications. Unlike in other imaging fields, human embryology and IVF have not yet leveraged artificial intelligence (AI) for unbiased, automated embryo assessment. We postulated that an AI approach trained on thousands of embryos can reliably predict embryo quality without human intervention. We implemented an AI approach based on deep neural networks (DNNs) to select highest quality embryos using a large collection of human embryo time-lapse images (about 50,000 images) from a high-volume fertility center in the United States. We developed a framework (STORK) based on Google's Inception model. STORK predicts blastocyst quality with an AUC of >0.98 and generalizes well to images from other clinics outside the US and outperforms individual embryologists. Using clinical data for 2182 embryos, we created a decision tree to integrate embryo quality and patient age to identify scenarios associated with pregnancy likelihood. Our analysis shows that the chance of pregnancy based on individual embryos varies from 13.8% (age ≥41 and poor-quality) to 66.3% (age <37 and good-quality) depending on automated blastocyst quality assessment and patient age. In conclusion, our AI-driven approach provides a reproducible way to assess embryo quality and uncovers new, potentially personalized strategies to select embryos.
Keywords: Image processing; Machine learning.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures



References
-
- Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States 1982-2010: data from the National Survey of Family Growth. Natl. Health Stat. Report. 2013;67:1–18. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

