Deep learning predicts hip fracture using confounding patient and healthcare variables
- PMID: 31304378
- PMCID: PMC6550136
- DOI: 10.1038/s41746-019-0105-1
Deep learning predicts hip fracture using confounding patient and healthcare variables
Abstract
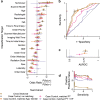
Hip fractures are a leading cause of death and disability among older adults. Hip fractures are also the most commonly missed diagnosis on pelvic radiographs, and delayed diagnosis leads to higher cost and worse outcomes. Computer-aided diagnosis (CAD) algorithms have shown promise for helping radiologists detect fractures, but the image features underpinning their predictions are notoriously difficult to understand. In this study, we trained deep-learning models on 17,587 radiographs to classify fracture, 5 patient traits, and 14 hospital process variables. All 20 variables could be individually predicted from a radiograph, with the best performances on scanner model (AUC = 1.00), scanner brand (AUC = 0.98), and whether the order was marked "priority" (AUC = 0.79). Fracture was predicted moderately well from the image (AUC = 0.78) and better when combining image features with patient data (AUC = 0.86, DeLong paired AUC comparison, p = 2e-9) or patient data plus hospital process features (AUC = 0.91, p = 1e-21). Fracture prediction on a test set that balanced fracture risk across patient variables was significantly lower than a random test set (AUC = 0.67, DeLong unpaired AUC comparison, p = 0.003); and on a test set with fracture risk balanced across patient and hospital process variables, the model performed randomly (AUC = 0.52, 95% CI 0.46-0.58), indicating that these variables were the main source of the model's fracture predictions. A single model that directly combines image features, patient, and hospital process data outperforms a Naive Bayes ensemble of an image-only model prediction, patient, and hospital process data. If CAD algorithms are inexplicably leveraging patient and process variables in their predictions, it is unclear how radiologists should interpret their predictions in the context of other known patient data. Further research is needed to illuminate deep-learning decision processes so that computers and clinicians can effectively cooperate.
Keywords: Computer science; Radiography; Statistics.
Conflict of interest statement
Competing interestsM.A.B. has received consulting fees from Whiteboard Coordinator, Inc. J.T.D. has received consulting fees or honoraria from Janssen Pharmaceuticals, GlaxoSmithKline, AstraZeneca, and Hoffman-La Roche. J.T.D. is a scientific advisor to L.A.M. Therapeutics and holds equity in NuMedii, Ayasdi, and Ontomics. M.L., M.V.M., and T.M.S. are employees of Verily Life Sciences.
Figures



References
-
- Ward, R. J. et al. ACR Appropriateness Criteria® Acute HipPain—Suspected Fracture. https://acsearch.acr.org/docs/3082587/Narrative/ (2018).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

