Conformational profile, vibrational assignments, NLO properties and molecular docking of biologically active herbicide1,1-dimethyl-3-phenylurea
- PMID: 31304416
- PMCID: PMC6600072
- DOI: 10.1016/j.heliyon.2019.e01987
Conformational profile, vibrational assignments, NLO properties and molecular docking of biologically active herbicide1,1-dimethyl-3-phenylurea
Abstract
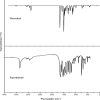
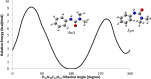
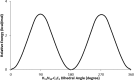
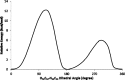
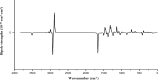
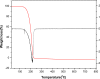
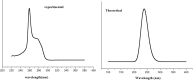
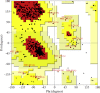
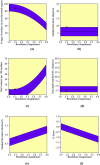
1,1-Dimethyl-3-phenylurea (known as fenuron) which is a phenyl urea-based widely used herbicide exhibits interesting structural and conformational properties and a notable biological activity. A detailed analysis on the vibrational, molecular and electronic characteristics of fenuron has been carried out. Potential energy scans (PESs) performed at the B3LYP/6-311++G(d,p) level of theory predicted two possible minima corresponding to the optimized anti and synforms resulting from the internal rotation about the N-C bond. The presence of an auxochrome together with the interaction with DMSO solvent exhibited a blue shift corresponding to the C=O orbitals. Delocalization of HOMO and LUMO orbital facilitated the charge transfer effect in the molecule. The calculated HOMO-LUMO energies, chemical potential, energy gap and global hardness suggested a low softness value for the compound while its biological activity was described by the value of electrophilicity. Chlorine substitution in the phenyl ring influenced the orbital delocalization for ortho and para substitutions but that of meta remained unaffected. NLO properties were noticed to increase due to chlorine substitution in the parent molecule. The docking results suggested that the compound exhibits an inhibitory activity against mitochondrial ubiquinol-cytochrome-c reductase and can be developed as a potential anticancer agent.
Keywords: DFT; Fenuron; Molecular docking; NBO; NLO; Organic chemistry; Pharmaceutical chemistry; Theoretical chemistry.
Figures




Similar articles
-
Quantum mechanical, spectroscopic and docking studies of 2-Amino-3-bromo-5-nitropyridine by Density Functional Method.Spectrochim Acta A Mol Biomol Spectrosc. 2017 Jun 15;181:153-163. doi: 10.1016/j.saa.2017.03.045. Epub 2017 Mar 19. Spectrochim Acta A Mol Biomol Spectrosc. 2017. PMID: 28359904
-
Molecular structure, vibrational, electronic and thermal properties of 4-vinylcyclohexene by quantum chemical calculations.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Jun 15;145:340-352. doi: 10.1016/j.saa.2015.03.043. Epub 2015 Mar 9. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25795608
-
Spectroscopic investigation (FT-IR and FT-Raman), vibrational assignments, HOMO-LUMO, NBO, MEP analysis and molecular docking study of 2-(4-hydroxyphenyl)-4,5-dimethyl-1H-imidazole 3-oxide.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Jul 5;146:307-22. doi: 10.1016/j.saa.2015.03.063. Epub 2015 Mar 10. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25819320
-
Assessment of long-range corrected and conventional DFT functional for the prediction of second--order NLO properties and other molecular properties of N-(2-cyanoethyl)-N-butylaniline--a vibrational spectroscopy study.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Jul 5;146:66-79. doi: 10.1016/j.saa.2015.03.048. Epub 2015 Mar 10. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25813164
-
FTIR, FT-RAMAN, NMR, spectra, normal co-ordinate analysis, NBO, NLO and DFT calculation of N,N-diethyl-4-methylpiperazine-1-carboxamide molecule.Spectrochim Acta A Mol Biomol Spectrosc. 2013 Nov;115:275-86. doi: 10.1016/j.saa.2013.06.011. Epub 2013 Jun 20. Spectrochim Acta A Mol Biomol Spectrosc. 2013. PMID: 23845985
Cited by
-
Detailed quantum mechanical, molecular docking, QSAR prediction, photovoltaic light harvesting efficiency analysis of benzil and its halogenated analogues.Heliyon. 2019 Nov 14;5(11):e02825. doi: 10.1016/j.heliyon.2019.e02825. eCollection 2019 Nov. Heliyon. 2019. PMID: 31763480 Free PMC article.
-
Excited-state electronic properties, structural studies, noncovalent interactions, and inhibition of the novel severe acute respiratory syndrome coronavirus 2 proteins in Ripretinib by first-principle simulations.J Mol Liq. 2021 Feb 15;324:115134. doi: 10.1016/j.molliq.2020.115134. Epub 2020 Dec 27. J Mol Liq. 2021. PMID: 33390634 Free PMC article.
-
Molecular docking, spectroscopic, and quantum chemical studies on aromatic heterocycle tetrakis(4-pyridyl)cyclobutane regioisomers: potential membrane-permeable inhibitors.J Mol Model. 2021 Feb 27;27(3):94. doi: 10.1007/s00894-021-04687-3. J Mol Model. 2021. PMID: 33638718
-
Synthesis, single crystal (XRD), Hirshfeld surface analysis, computational study (DFT) and molecular docking studies of (E)-4-((2-hydroxy-3,5-diiodobenzylidene)amino)-N-(pyrimidine)-2-yl) benzenesulfonamide.Heliyon. 2021 Aug 6;7(8):e07724. doi: 10.1016/j.heliyon.2021.e07724. eCollection 2021 Aug. Heliyon. 2021. PMID: 34458601 Free PMC article.
-
Detailed molecular structure (XRD), conformational search, spectroscopic characterization (IR, Raman, UV, fluorescence), quantum mechanical properties and bioactivity prediction of a pyrrole analogue.Heliyon. 2020 Jun 3;6(6):e04106. doi: 10.1016/j.heliyon.2020.e04106. eCollection 2020 Jun. Heliyon. 2020. PMID: 32529077 Free PMC article.
References
-
- Gangwar S.K., Rafiquee M.Z.A. Kinetics of the alkaline hydrolysis of fenuron in aqueous and micellar media. Int. J. Chem. Kinet. 2007;39:638–644.
-
- Oturan M.A., Edelahi M.C., Oturan N., El K., Aaron J. Environmental kinetics of oxidative degradation/mineralization pathways of the phenylurea herbicides diuron, monuron and fenuron in water during the application of the electro-Fenton process. Appl. Catal. B Environ. 2010;97:82–89.
-
- Tahmasseb L.A., Nelieu S., Kerhoas L., Einhorn J. Ozonation of chlorophenylurea pesticides in water, reaction monitoring and degradation pathways. Sci. Total Environ. 2002;291:33–44. - PubMed
-
- Gallard H., De Laat J. Kinetics of oxidation of chlorobenzenes and phenylureas by Fe(II)/H2O2 and Fe(III)H2O2, evidence of reduction and oxidation reactions of intermediates by Fe(II) or Fe(III) Chemosphere. 2001;42:405–413. - PubMed
-
- Mazellier D.L.J., Busset P., Delmont A. A comparison of fenuron degradation by hydroxyl and carbonate radicals in aqueous solution. Water Res. 2007;41:4585–4594. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

