Pathology of pancreatic cancer
- PMID: 31304427
- PMCID: PMC6624347
- DOI: 10.21037/tgh.2019.06.02
Pathology of pancreatic cancer
Abstract
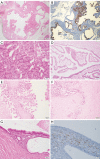
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive malignancy and estimated to become the second leading cause of cancer-related deaths by 2030. Although overall 5-year survival rates have constantly remained below 10% for the last decades, several key points important for accurate patient stratification have emerged during recent years. These key points include a highly standardized gross examination of PDAC resection specimens, using an axial slicing technique and inking of the circumferential resection margin (CRM), as well as a meticulous microscopic examination, taking into account the prognostic relevance of factors such as the exact resection status (R0 vs. R1 1-mm vs. R1 resection), histopathological tumor grading and the so-called lymph node ratio (LNR). With increasing use of neoadjuvant therapy in PDAC, tumor regression grading (TRG) for PDAC is currently rising in relevance in order to stratify and manage pre-operatively treated PDAC patients. As all current TRG systems for PDAC are unsatisfactory, new standardized international protocols are urgently needed. Several morphological subtypes of PDAC exist, some of which share the same molecular background with classical PDAC, while others are characterized by a distinct molecular pathogenesis. While some show a prognosis similar to classical PDAC, other subtypes stand out due to a better or even worse prognosis than classical PDAC. Prognostic relevant molecular subtypes of PDAC have been proposed as well, however, limitations of used cohorts and the lacking correlation of molecular subtypes with histomorphological subtypes limit the translation of these findings into valuable clinical applications. Lastly, several macroscopic and microscopic precursor lesions of PDAC have been described in genetically engineered mouse models (GEMM) and humans in recent times, providing further insight into PDAC carcinogenesis. In addition, improved diagnosis of PDAC precursors represents a chance to select patients for resection before invasive PDAC is present.
Keywords: Histomorphological variants; molecular subtypes; neoadjuvant treatment; pancreatic ductal adenocarcinoma (PDAC); precursor lesions; standardized pathology report.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
