Transnuclear mice reveal Peyer's patch iNKT cells that regulate B-cell class switching to IgG1
- PMID: 31304630
- PMCID: PMC6627243
- DOI: 10.15252/embj.2018101260
Transnuclear mice reveal Peyer's patch iNKT cells that regulate B-cell class switching to IgG1
Abstract
Tissue-resident iNKT cells maintain tissue homeostasis and peripheral surveillance against pathogens; however, studying these cells is challenging due to their low abundance and poor recovery from tissues. We here show that iNKT transnuclear mice, generated by somatic cell nuclear transfer, have increased tissue resident iNKT cells. We examined expression of PLZF, T-bet, and RORγt, as well as cytokine/chemokine profiles, and found that both monoclonal and polyclonal iNKT cells differentiated into functional subsets that faithfully replicated those seen in wild-type mice. We detected iNKT cells from tissues in which they are rare, including adipose, lung, skin-draining lymph nodes, and a previously undescribed population in Peyer's patches (PP). PP-NKT cells produce the majority of the IL-4 in Peyer's patches and provide indirect help for B-cell class switching to IgG1 in both transnuclear and wild-type mice. Oral vaccination with α-galactosylceramide shows enhanced fecal IgG1 titers in iNKT cell-sufficient mice. Transcriptional profiling reveals a unique signature of PP-NKT cells, characterized by tissue residency. We thus define PP-NKT as potentially important for surveillance for mucosal pathogens.
Keywords: IL-4, tissue-resident iNKT cells; Peyer's patches; oral vaccines; transnuclear mice.
© 2019 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Diagram representing the placement of various tissues analyzed for iNKT cells. mLN = mesenteric lymph node; sdLN = skin‐draining lymph node.
Relative iNKT cell yield in various tissues from TN mouse lines compared to C57BL/6 mouse lines. Tissues were isolated from indicated C57BL/6 or iNKT TN mouse lines and stained with anti‐CD3 and CD1d‐(PBS57)‐tetramer.
Spleen, mLN, sdLN, liver, adipose, and lung lymphocytes from Jα18−/− or Vα14 mice were stimulated in vitro with RAW‐CD1d cells and 1 μg α‐GalCer. An additional sample of Vα14 lymphocytes from each organ was plated with RAW‐CD1d cells but no α‐GalCer. Supernatants were collected after 24 h and cytokine concentration determined by cytokine bead array. Error bars are SD of mean values from three different mice per group. Results shown are representative of two independent experiments where n = 3 biological replicates.

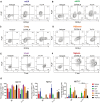
- A–F
Lymphocytes from the indicated tissues of C57BL/6 and Vα14 mice were stained with anti‐CD3 and CD1d‐(PBS57)‐tetramer, before they were fixed, permeabilized, and stained with antibodies to T‐bet, RORγt, and PLZF. Results shown are gated on CD3+CD1d‐tetramer+ cells.
- G
The percentage of CD3+ CD1d‐tetramer+ iNKT cells in each organ that stained positively for PLZF, T‐bet, and RORγt are shown. **P < 0.01, Mann–Whitney test. Error bars are SD.
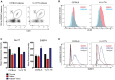
Flow cytometry analysis of iNKT cell abundance in white adipose tissue from a Vα14 TN mouse.
Spleen cells and stromal vascular fractions of white adipose tissue from Vα14 TN or C57BL/6 mice were stained intracellularly with anti‐PLZF and analyzed by flow cytometry. Histograms shown are gated on CD1d‐(PBS57)‐tetramer+ CD3+ cells.
Thymus, spleen, and adipose tissue were harvested from C57BL/6 mice and Vα14 TN mice. Cell suspensions were stained with antibodies to CD3, Nur77, E4BP4, and CD1d‐(PBS57)‐tetramer and analyzed by flow cytometry. Mean fluorescence intensity of Nur77 and E4BP4 staining after gating on iNKT cells is shown. N = 3 per group. Error bars are SEM.
Representative histograms of E4BP4 staining are shown. Plots are gated on total CD3+ cells.
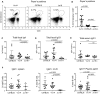
- A
Lymphocytes from Peyer's patches of C57BL/6 and Vα14 mice were stained with anti‐CD3 and CD1d‐(PBS57)‐tetramer.
- B
Percentage of lymphocytes that were CD3+CD1d‐tetramer+ iNKT cells among Peyer's patches of C57BL/6, Vα14, and Jα18−/− mice are shown. Mann–Whitney test. Error bars are SD. C57BL/6 n = 22; Vα14 n = 16; Jα18−/− n = 8.
- C–E
Mice were analyzed for total fecal IgA (C), total fecal IgG1 (D), or total serum IgG1 (E) by ELISA. Mann–Whitney test. Error bars are SD. C57BL/6 n = 14; Vα14 n = 11; Jα18−/− n = 13.
- F
Percentages of total B cells that were IgG1+ in the spleen, mLN, and Peyer's patches of C57BL/6, Vα14, and Jα18−/− mice are shown. Mann–Whitney test. Error bars are SD. C57BL/6 n = 15; Vα14 n = 15; Jα18−/− n = 5.

Stool collected from C57BL/6 and CD1d−/− mice was analyzed by ELISA for IgG1 as described in Fig 3. Mice were age‐ and sex‐matched and housed in the Longwood Center facility. C57BL/6 n = 18; CD1d−/− n = 5. Mann–Whitney test. Error bars are SD.
Spleen, mLN, and PP cells were harvested from wild‐type or CD1d−/− mice, stained with antibodies to B220 and IgG1, and analyzed by flow cytometry. Mice were age‐ and sex‐matched and housed in the Longwood Center facility. C57BL/6 n = 5; CD1d−/− n = 3. Mann–Whitney test. Error bars are SD.
Analysis was performed identically to that shown in Fig 3F, except that mice were housed in the Smith Building at Dana‐Farber Cancer Institute prior to moving to the Longwood Center at Dana‐Farber Cancer Institute. Results in Fig 3 are entirely from mice housed in the Longwood Center facility. C57BL/6 n = 10; Vα14−/− n = 12; Jα18−/− n = 3. Mann–Whitney test. Error bars are SD.
Culture supernatants from Fig 5D and E were measured by ELISA for IgM. np = not performed. Mann–Whitney test. Error bars are SD.

- A
CD1d‐(PBS57)‐tetramer+ CD3+ cells were sorted from spleens or PP of 3 different Vα14 TN mice along with CD4+CD3+CD1d‐tetramer− cells from PP (PP CD4). RNAseq was performed.
- B
Heatmap of FPKM values for the indicated Tfh genes across each RNAseq sample.
- C, D
Spleen, mLN, and PP lymphocytes from Vα14 and C57BL/6 mice were stimulated with PMA and ionomycin. Lymphocytes were stained with anti‐CD3, CD1d‐(PBS57)‐tetramer, anti‐IL‐4, and anti‐IFNγ. Percentages of iNKT cells and non‐iNKT T cells within the population of CD3+IL‐4+ cells and CD3+IFNγ+ cells, n = 5 mice for C57BL/6 group and n = 4 mice for Vα14 group. Error bars are SD.
- E
Vα14 TN mice were administered α‐GalCer either 2 μg intravenously or 5 μg by oral gavage. Mice were given brefeldin A intraperitoneally after 30 min, and tissues were harvested 3 h later. Cells from spleen, mesenteric lymph node, and Peyer's patches of Vα14 TN mice were permeabilized, fixed, and stained with antibodies to IL‐4, IFNγ, and IL‐17. Plots shown are gated on CD1d‐(PBS57)‐tetramer+ CD3+ iNKT cells.
- F
Quantification of data from (E), n = 2 mice per group. Mann–Whitney test. Error bars are SEM.
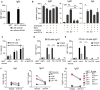
- A
Pooled spleen and LN cells from a Vα14 TN mouse were cocultured with wild‐type B cells with or without 1 μg α‐GalCer and with or without agnostic anti‐CD40. IgM was measured by ELISA of culture supernatants 4 days later.
- B
Pooled spleen and LN cells from a Vα14 TN mouse were cocultured with anti‐CD40‐activated wild‐type B cells with or without 1 μg α‐GalCer and with blocking antibody to CD1d (1 μg/ml, clone 1B1) or isotype control as indicated. IgM, IgG1, and IgA were measured by ELISA of 4‐day culture supernatants.
- C–G
Spleen, mLN, or PP cells from Vα14 TN iNKT mice and PP cells from an IL‐4−/− mouse were cocultured with anti‐CD40‐activated wild‐type or CD1d−/− B cells. 1 μg α‐GalCer was added to the cultures to specifically activate iNKT cells. Blocking antibodies to IL‐4 or isotype were added as indicated. IL‐4 (C) and IgG1 (D, E) were measured by ELISA of culture supernatants. IgG1+ and IgA+ class‐switched B cells were enumerated by flow cytometry after 4 days of coculture (F, G). Representative of two independent experiments. np = not performed.

- A
Mice were administered ovalbumin by oral gavage, with or without α‐GalCer at the indicated time points, at the indicated amounts. Mice used were Jα18−/− (n = 10 total), iNKT transnuclear (n = 7 total), or littermate controls of iNKT TN mice that did not inherit the Vα14 allele (B6, n = 12 total).
- B, C
OVA‐specific antibody titers from feces and blood were determined by ELISA from the mice in (A). Shown are optical density values for samples collected on Day 52 post‐vaccination (7 days after the final boost). Error bars are SD.
- D
Cells from spleen, mesenteric lymph node, and Peyer's patches of Vα14 TN mice were permeabilized, fixed, and stained with the indicated antibodies. Plots shown are gated on CD1d‐(PBS57)‐tetramer+ CD3+ iNKT cells.
- E
Quantification of data from (D), n = 3 mice per group. Mann–Whitney test. Error bars are SEM. *P < 0.05.
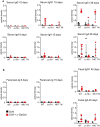
Anti‐ovalbumin antibodies were detected by ELISA from serum of immunized mice as indicated. Secondary antibodies to either IgG1 or IgA were used for detection.
Fecal pellets were collected from immunized mice and diluted in water at 10 mg stool/ml. Anti‐ovalbumin antibodies were detected by ELISA as indicated. Secondary antibodies to pan‐Ig, IgG1, or IgA were used for detection.

Jα18−/− mice were lightly irradiated with 100 Gy prior to receiving either no cell transfer (bottom panels) or transfer of 10 million pooled spleen and LN cells from Va14 TN mice. Input cells were verified by flow cytometry to contain > 30% CD1d‐tetramer+ cells. Two weeks post‐transfer, the indicated tissues were harvested and analyzed by flow cytometry. Representative of n = 5 recipient mice per group. Representative of three independent experiments.
Lymphocyte preparations from the indicated tissues of Jα18−/− mice from (A) were cocultured with RAWd cells and 1 μg α‐GalCer as in Fig 1. IL‐4 was measured by ELISA of 48‐h culture supernatants. Error bars are SD of triplicate samples.

Principal component analysis of RNAseq samples from biological replicates of PP‐iNKT, spleen iNKT, and PP CD4+ cells
Differential expression analysis comparing transcriptomes of spleen iNKT cells versus PP‐iNKT cells.
Heatmap of FPKM values from genes uniquely upregulated in PP‐iNKT cells as compared to both spleen iNKT and PP CD4 cells. Red indicates higher expression, and blue indicates lower expression.
Spleen and PP cells from Vα14 TN mice were stained with the indicated antibodies, analyzed by flow cytometry, and gated on CD1d‐(PBS57)‐tetramer+ CD3+ cells.
Quantification of data from (D), n = 11 mice per group. Mann–Whitney test. Error bars are SEM.
Similar articles
-
Monoclonal Invariant NKT (iNKT) Cell Mice Reveal a Role for Both Tissue of Origin and the TCR in Development of iNKT Functional Subsets.J Immunol. 2017 Jul 1;199(1):159-171. doi: 10.4049/jimmunol.1700214. Epub 2017 Jun 2. J Immunol. 2017. PMID: 28576977 Free PMC article.
-
Induction of IgA B cell differentiation of bone marrow-derived B cells by Peyer's patch autoreactive helper T cells.Immunol Invest. 1995 Aug;24(5):701-11. doi: 10.3109/08820139509060699. Immunol Invest. 1995. PMID: 8543335
-
Alternate mucosal immune system: organized Peyer's patches are not required for IgA responses in the gastrointestinal tract.J Immunol. 2000 May 15;164(10):5184-91. doi: 10.4049/jimmunol.164.10.5184. J Immunol. 2000. PMID: 10799877
-
Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. II. Terminal differentiation of postswitch sIgA-bearing Peyer's patch B cells.J Exp Med. 1983 Sep 1;158(3):649-69. doi: 10.1084/jem.158.3.649. J Exp Med. 1983. PMID: 6604126 Free PMC article.
-
IL-21: a new player in the control of isotype switch in Peyer's patches.J Leukoc Biol. 2009 May;85(5):739-43. doi: 10.1189/jlb.0109045. J Leukoc Biol. 2009. PMID: 19406834 Review.
Cited by
-
IFN-γ-dependent regulation of intestinal epithelial homeostasis by NKT cells.Cell Rep. 2024 Dec 24;43(12):114948. doi: 10.1016/j.celrep.2024.114948. Epub 2024 Nov 23. Cell Rep. 2024. PMID: 39580798 Free PMC article.
-
Invariant natural killer T cells balance B cell immunity.Immunol Rev. 2021 Jan;299(1):93-107. doi: 10.1111/imr.12938. Epub 2021 Jan 12. Immunol Rev. 2021. PMID: 33438287 Free PMC article. Review.
-
Loss of Mitochondrial Tusc2/Fus1 Triggers a Brain Pro-Inflammatory Microenvironment and Early Spatial Memory Impairment.Int J Mol Sci. 2024 Jul 5;25(13):7406. doi: 10.3390/ijms25137406. Int J Mol Sci. 2024. PMID: 39000512 Free PMC article.
References
-
- Brennan PJ, Brigl M, Brenner MB (2013) Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 13: 101–117 - PubMed
-
- Bry L, Brenner MB (2004) Critical role of T cell‐dependent serum antibody, but not the gut‐associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J Immunol 172: 433–441 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01 AI113046/AI/NIAID NIH HHS/United States
- F31 AI138353/AI/NIAID NIH HHS/United States
- R01 AI134861/AI/NIAID NIH HHS/United States
- RO1 AI113046/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- 1F31 AI138353-01/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

