Resting-State fMRI Networks in Children with Tuberous Sclerosis Complex
- PMID: 31304656
- PMCID: PMC7020733
- DOI: 10.1111/jon.12653
Resting-State fMRI Networks in Children with Tuberous Sclerosis Complex
Abstract
Background and purpose: There are no published studies examining resting state networks (RSNs) and their relationship with neurodevelopmental metrics in tuberous sclerosis complex (TSC). We aimed to identify major resting-state functional magnetic resonance imaging (rs-fMRI) networks in infants with TSC and correlate network analyses with neurodevelopmental assessments, autism diagnosis, and seizure history.
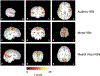
Methods: Rs-fMRI data from 34 infants with TSC, sedated with propofol during the scan, were analyzed to identify auditory, motor, and visual RSNs. We examined the correlations between auditory, motor, and visual RSNs at approximately 11.5 months, neurodevelopmental outcome at approximately 18.5 months, and diagnosis of autism spectrum disorders at approximately 36 months of age.
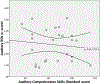
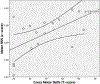
Results: RSNs were obtained in 76.5% (26/34) of infants. We observed significant negative correlations between auditory RSN and auditory comprehension test scores (p = .038; r = -.435), as well as significant positive correlations between motor RSN and gross motor skills test scores (p = .023; r = .564). Significant positive correlations between motor RSNs and gross motor skills (p = .012; r = .754) were observed in TSC infants without autism, but not in TSC infants with autism, which could suggest altered motor processing. There were no significant differences in RSNs according to seizure history.
Conclusions: Negative correlation between auditory RSN, as well as positive correlation between motor RSN and developmental outcome measures might reflect different brain mechanisms and, when identified, may be helpful in predicting later function. A larger study of TSC patients with a healthy control group is needed before auditory and motor RSNs could be considered as neurodevelopmental outcome biomarkers.
Keywords: Autism spectrum disorders; propofol; resting state functional resonance imaging; resting state networks; tuberous sclerosis complex.
© 2019 by the American Society of Neuroimaging.
Figures

References
-
- Curatolo P, Bombardieri R. Tuberous sclerosis. Handb Clin Neurol 2008;87:129–51. - PubMed
-
- Hallett L, Foster T, Liu Z, Blieden M, Valentim J. Burden of disease and unmet needs in tuberous sclerosis complex with neurological manifestations: Systematic review. Curr Med Res Opin 2011;27:1571–83. - PubMed
-
- Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci 1991;615:125–7. - PubMed
-
- Au KS, Williams AT, Roach ES, Batchelor L, Sparagana SP, Delgado MR, et al. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the united states. Genet Med 2007;9:88–100. - PubMed
-
- Crino PB. Evolving neurobiology of tuberous sclerosis complex. Acta Neuropathol 2013;125:317–32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

