Systemic neoadjuvant chemotherapy in modern pancreatic cancer treatment: a systematic review and meta-analysis
- PMID: 31304767
- PMCID: PMC6667953
- DOI: 10.1308/rcsann.2019.0060
Systemic neoadjuvant chemotherapy in modern pancreatic cancer treatment: a systematic review and meta-analysis
Abstract
Background: Pancreatic ductal adenocarcinoma remains a disease with a poor prognosis despite advances in surgery and systemic therapies. Neoadjuvant therapy strategies are a promising alternative to adjuvant chemotherapy. However, their role remains controversial. This meta-analysis aims to clarify the benefits of neoadjuvant therapy in resectable pancreatic ductal adenocarcinoma.
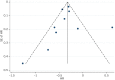
Methods: Eligible studies were identified from MEDLINE, Embase, Web of Science and the Cochrane Library. Studies comparing neoadjuvant therapy with a surgery first approach (with or without adjuvant therapy) in resectable pancreatic ductal adenocarcinoma were included. The primary outcome assessed was overall survival. A random-effects meta-analysis was performed, together with pooling of unadjusted Kaplan-Meier curve data.
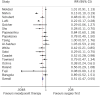
Results: A total of 533 studies were identified that analysed the effect of neoadjuvant therapy in pancreatic ductal adenocarcinoma. Twenty-seven studies were included in the final data synthesis. Meta-analysis suggested beneficial effects of neoadjuvant therapy with prolonged survival compared with a surgery-first approach, (hazard ratio 0.72, 95% confidence interval 0.69-0.76). In addition, R0 resection rates were significantly higher in patients receiving neoadjuvant therapy (relative risk 0.51, 95% confidence interval 0.47-0.55). Individual patient data analysis suggested that overall survival was better for patients receiving neoadjuvant therapy (P = 0.008).
Conclusions: Current evidence suggests that neoadjuvant chemotherapy has a beneficial effect on overall survival in resectable pancreatic ductal adenocarcinoma in comparison with upfront surgery and adjuvant therapy. Further trials are needed to address the need for practice change.
Keywords: Cancer; Neoadjuvant; Pancreas; Surgery.
Figures



Similar articles
-
Network meta-analysis comparing neoadjuvant chemoradiation, neoadjuvant chemotherapy and upfront surgery in patients with resectable, borderline resectable, and locally advanced pancreatic ductal adenocarcinoma.Radiat Oncol. 2019 Jul 10;14(1):120. doi: 10.1186/s13014-019-1330-0. Radiat Oncol. 2019. PMID: 31291998 Free PMC article.
-
Neoadjuvant treatment versus upfront surgery in borderline resectable and resectable pancreatic ductal adenocarcinoma: meta-analysis.BJS Open. 2025 Mar 4;9(2):zrae172. doi: 10.1093/bjsopen/zrae172. BJS Open. 2025. PMID: 40126570 Free PMC article.
-
Low lymphocyte monocyte ratio after neoadjuvant therapy predicts poor survival after pancreatectomy in patients with borderline resectable pancreatic cancer.Surgery. 2019 Jun;165(6):1151-1160. doi: 10.1016/j.surg.2018.12.015. Epub 2019 Feb 11. Surgery. 2019. PMID: 30765142
-
Prognostic factors in localized pancreatic ductal adenocarcinoma after neoadjuvant therapy and resection: a systematic review and meta-analysis.J Natl Cancer Inst. 2025 May 1;117(5):840-867. doi: 10.1093/jnci/djae294. J Natl Cancer Inst. 2025. PMID: 39563429
-
Increased neutrophil-to-lymphocyte ratio after neoadjuvant therapy is associated with worse survival after resection of borderline resectable pancreatic ductal adenocarcinoma.Surgery. 2016 Nov;160(5):1288-1293. doi: 10.1016/j.surg.2016.04.039. Epub 2016 Jul 20. Surgery. 2016. PMID: 27450715 Free PMC article.
Cited by
-
PACT-UK (PAncreatic Cancer reporting Template-UK): a cross-specialty multi-institutional consensus panel development of a standardised radiological reporting proforma for pancreatic cancer.BMJ Oncol. 2023 Nov 20;2(1):e000055. doi: 10.1136/bmjonc-2023-000055. eCollection 2023. BMJ Oncol. 2023. PMID: 39886489 Free PMC article.
-
Present status and perspective of perioperative chemotherapy for patients with resectable pancreatic cancer in Japan.Glob Health Med. 2022 Feb 28;4(1):14-20. doi: 10.35772/ghm.2021.01015. Glob Health Med. 2022. PMID: 35291202 Free PMC article. Review.
-
Current controversies and advances in the management of pancreatic adenocarcinoma.World J Gastrointest Oncol. 2021 Jun 15;13(6):472-494. doi: 10.4251/wjgo.v13.i6.472. World J Gastrointest Oncol. 2021. PMID: 34163568 Free PMC article. Review.
-
Comparison of the upfront surgery and neoadjuvant therapy in resectable and borderline resectable pancreatic cancer: an updated systematic review and meta-analysis.Updates Surg. 2024 Jan;76(1):1-15. doi: 10.1007/s13304-023-01626-0. Epub 2023 Aug 28. Updates Surg. 2024. PMID: 37639177
-
Essential updates 2018/2019: Current topics in the surgical treatment of pancreatic ductal adenocarcinoma.Ann Gastroenterol Surg. 2020 Aug 9;5(1):7-23. doi: 10.1002/ags3.12379. eCollection 2021 Jan. Ann Gastroenterol Surg. 2020. PMID: 33532676 Free PMC article. Review.
References
-
- Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993; (1): 68–733. - PubMed
-
- Neoptolemos JP, Stocken DD, Friess H et al. . A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004; (12): 1,200–1,210. - PubMed
-
- Neoptolemos JP, Palmer DH, Ghaneh P et al. . Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017; (10073): 1,011–1,024. - PubMed
-
- Edeline J, Bonnetain F, Phelip JM et al. . Gemox versus surveillance following surgery of localized biliary tract cancer: Results of the PRODIGE 12-ACCORD 18 (UNICANCER GI) phase III trial. J Clin Oncol 2017; (4 Suppl): 225. - PubMed
-
- Aloia TA, Aloia TE, Lee JE et al. . Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg 2007; (3): 347–355. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

