Adalimumab for nail psoriasis: efficacy and safety over 52 weeks from a phase-3, randomized, placebo-controlled trial
- PMID: 31304993
- PMCID: PMC6899987
- DOI: 10.1111/jdv.15793
Adalimumab for nail psoriasis: efficacy and safety over 52 weeks from a phase-3, randomized, placebo-controlled trial
Abstract
Background: Few clinical trials have evaluated long-term treatment of nail psoriasis with biologics.
Objective: Safety and efficacy of adalimumab [ADA; Humira AbbVie Inc, North Chicago, IL, USA)] long-term treatment (52 weeks) was evaluated in a phase-3, randomized trial in patients with moderate-to-severe plaque psoriasis and concomitant moderate-to-severe fingernail psoriasis. Results from the first 26 weeks (Period A) have been reported.
Methods: Patients receiving 40 mg ADA every other week or placebo in Period A, continued with or switched to 40 mg ADA every-other-week treatment in the subsequent 26-week open-label extension (OLE) period. Main efficacy evaluations were ≥75% improvement in total-fingernail modified Nail Psoriasis Severity Index (mNAPSI 75) and achievement of Physician's Global Assessment for Fingernail Psoriasis of clear or minimal disease (PGA-F 0/1) with a ≥2-grade improvement from baseline, across the trial for patients who continued ADA from Period A through the OLE (Continuous-ADA Population). Safety was evaluated during the OLE and for patients receiving ADA at any time during the study (All-ADA Population).
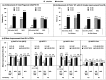
Results: Of the 217 patients initially randomized in Period A, 188 (86.6%; 94 in each treatment group) entered the OLE after completion of or early escape from Period A. For the Continuous-ADA Population (N = 109), endpoint achievement rates improved from OLE entry (Week 26) to Week 52, including total-fingernail mNAPSI 75 (47.4-54.5%); PGA-F 0/1 (51.1-55.6%) and total-fingernail mNAPSI = 0 (6.6-17.9%). Serious adverse event and serious infection rates for the All-ADA Population (N = 203) were 6.9% and 3.4%, respectively.
Conclusions: In this population of psoriasis patients with concomitant, moderate-to-severe nail psoriasis, long-term efficacy and improvement in signs and symptoms of nail disease were demonstrated after every-other-week ADA treatment, including incremental improvements in rate of total clearance of nail disease. No new safety risks were identified for patients receiving at least one ADA dose across 52 weeks.
© 2019 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures



References
-
- de Jong EM, Seegers BA, Gulinck MK et al Psoriasis of the nails associated with disability in a large number of patients: results of a recent interview with 1,728 patients. Dermatology 1996; 193: 300–303. - PubMed
-
- Augustin M, Reich K, Blome C et al Nail psoriasis in Germany: epidemiology and burden of disease. Br J Dermatol 2010; 163: 580–585. - PubMed
-
- Lawry M. Biological therapy and nail psoriasis. Dermatol Ther 2007; 20: 60–67. - PubMed
-
- Richert B, Caucanas M. Epidemiology of nail psoriasis In Rigopoulos D, Tosti A, eds. Nail Psoriasis: A to Z, Springer, Cham, Heidelberg, New York, Dordrecht, London, 2014: 1–8.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

