Current and new rotavirus vaccines
- PMID: 31305493
- PMCID: PMC6749957
- DOI: 10.1097/QCO.0000000000000572
Current and new rotavirus vaccines
Abstract
Purpose of review: As of 2019, four rotavirus vaccines have been prequalified by the WHO for use worldwide. This review highlights current knowledge regarding rotavirus vaccines available, and provides a brief summary of the rotavirus vaccine pipeline.
Recent findings: Data generated from use of currently available products supports their effectiveness and impact in diverse settings. Rotavirus vaccines have a favorable risk-benefit profile, but previous associations of rotavirus vaccination with intussusception necessitate continued monitoring for this rare but serious adverse event. Implementation of rotavirus vaccines was jeopardized in late 2018 and 2019 by a shortage of vaccine supply. Fortunately, with the prequalification of two additional vaccines in 2018, countries have increased choice in products with different characteristics, pricing, and implementation strategies. Other vaccines currently in development may open up further immunization strategies, such as neonatal vaccination schedules or parenteral administration.
Summary: Rotavirus vaccines have demonstrated impact in reducing diarrheal morbidity and mortality worldwide. As countries begin to introduce the newly prequalified vaccines, additional data will become available on the safety and effectiveness of those products. Products in the pipeline have distinct profiles and could be an essential part of the expansion of rotavirus vaccine use worldwide.
Figures

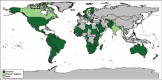
References
-
- Troeger C, Khalil IA, Rao PC, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr 2018; 172:958–965. - PMC - PubMed
-
This article, drawing on the Global Burden of Disease Study, provides updated estimates of annual rotavirus-specific mortality and morbidity, as well as estimated deaths averted by rotavirus vaccination, by country.
-
- Rotavirus vaccines. WHO position paper - January 2013. Wkly Epidemiol Rec 2013; 88:49–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

