Prevalence of Biologically vs Clinically Defined Alzheimer Spectrum Entities Using the National Institute on Aging-Alzheimer's Association Research Framework
- PMID: 31305929
- PMCID: PMC6632154
- DOI: 10.1001/jamaneurol.2019.1971
Prevalence of Biologically vs Clinically Defined Alzheimer Spectrum Entities Using the National Institute on Aging-Alzheimer's Association Research Framework
Abstract
Importance: A National Institute on Aging-Alzheimer's Association (NIA-AA) workgroup recently published a research framework in which Alzheimer disease is defined by neuropathologic or biomarker evidence of β-amyloid plaques and tau tangles and not by clinical symptoms.
Objectives: To estimate the sex- and age-specific prevalence of 3 imaging biomarker-based definitions of the Alzheimer disease spectrum from the NIA-AA research framework and to compare these entities with clinically defined diagnostic entities commonly linked with Alzheimer disease.
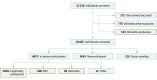
Design, setting, and participants: The Mayo Clinic Study of Aging (MCSA) is a population-based cohort study of cognitive aging in Olmsted County, Minnesota. The MCSA in-person participants (n = 4660) and passively ascertained (ie, through the medical record rather than in-person) individuals with dementia (n = 553) aged 60 to 89 years were included. Subsets underwent amyloid positron emission tomography (PET) (n = 1524) or both amyloid and tau PET (n = 576). Therefore, this study included 3 nested cohorts examined between November 29, 2004, and June 5, 2018. Data were analyzed between February 19, 2018, and March 26, 2019.
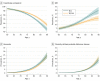
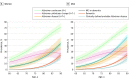
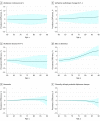
Main outcomes and measures: The sex- and age-specific prevalence of the following 3 biologically defined diagnostic entities was estimated: Alzheimer continuum (abnormal amyloid regardless of tau status), Alzheimer pathologic change (abnormal amyloid but normal tau), and Alzheimer disease (abnormal amyloid and tau). These were compared with the prevalence of 3 clinically defined diagnostic groups (mild cognitive impairment or dementia, dementia, and clinically defined probable Alzheimer disease).
Results: The median (interquartile range) age was 77 (72-83) years in the clinical cohort (n = 5213 participants), 77 (70-83) years in the amyloid PET cohort (n = 1524 participants), and 77 (69-83) years in the tau PET cohort (n = 576 participants). There were roughly equal numbers of women and men. The prevalence of all diagnostic entities (biological and clinical) increased rapidly with age, with the exception of Alzheimer pathologic change. The prevalence of biological Alzheimer disease was greater than clinically defined probable Alzheimer disease for women and men. Among women, these values were 10% (95% CI, 6%-14%) vs 1% (95% CI, 1%-1%) at age 70 years and 33% (95% CI, 25%-41%) vs 10% (95% CI, 9%-12%) at age 85 years (P < .001). Among men, these values were 9% (95% CI, 5%-12%) vs 1% (95% CI, 0%-1%) at age 70 years and 31% (95% CI, 24%-38%) vs 9% (95% CI, 8%-11%) at age 85 years (P < .001). The only notable difference by sex was a greater prevalence of the mild cognitive impairment or dementia clinical category among men than women.
Conclusions and relevance: Results of this study suggest that biologically defined Alzheimer disease is more prevalent than clinically defined probable Alzheimer disease at any age and is 3 times more prevalent at age 85 years among both women and men. This difference is mostly driven by asymptomatic individuals with biological Alzheimer disease. These findings illustrate the magnitude of the consequences on public health that potentially exist by intervening with disease-specific treatments to prevent symptom onset.
Conflict of interest statement
Figures
Comment in
-
Alzheimer Disease, Biomarkers, and Clinical Symptoms-Quo Vadis?-Reply.JAMA Neurol. 2020 Mar 1;77(3):394. doi: 10.1001/jamaneurol.2019.4962. JAMA Neurol. 2020. PMID: 32011648 No abstract available.
-
Alzheimer Disease, Biomarkers, and Clinical Symptoms-Quo Vadis?JAMA Neurol. 2020 Mar 1;77(3):393-394. doi: 10.1001/jamaneurol.2019.4959. JAMA Neurol. 2020. PMID: 32011649 No abstract available.
Comment on
-
The Challenge of Defining Alzheimer Disease Based on Biomarkers in the Absence of Symptoms.JAMA Neurol. 2019 Oct 1;76(10):1143-1144. doi: 10.1001/jamaneurol.2019.1667. JAMA Neurol. 2019. PMID: 31305888 No abstract available.
References
-
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. doi:10.1212/WNL.34.7.939 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

